Question
Triple-point coordinates for carbon dioxide are T t = 216.55 K and P t = 5.170 bar. Hence, CO 2 has no normal boiling point.
Triple-point coordinates for carbon dioxide are Tt = 216.55 K and Pt = 5.170 bar. Hence, CO2 has no normal boiling point. (Why?) Nevertheless, one can define a hypothetical normal boiling point by extrapolation of the vapor-pressure curve.
(a) Use the Lee/Kesler correlation for Prsat in conjunction with the triple-point coordinates to estimate ? for CO2. Compare it with the value in Table B.1.
(b) Use the Lee/Kesler correlation to estimate the hypothetical normal boiling point for CO2. Comment on the likely reasonableness of this result.
Table B.1: Characteristic Properties of Pure Species
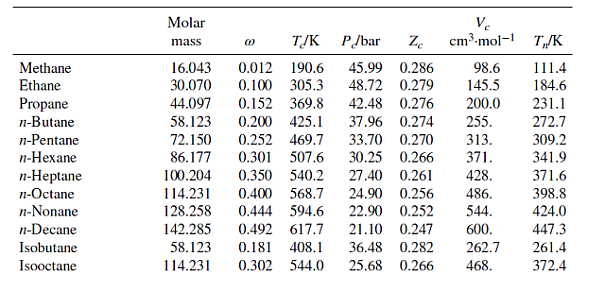
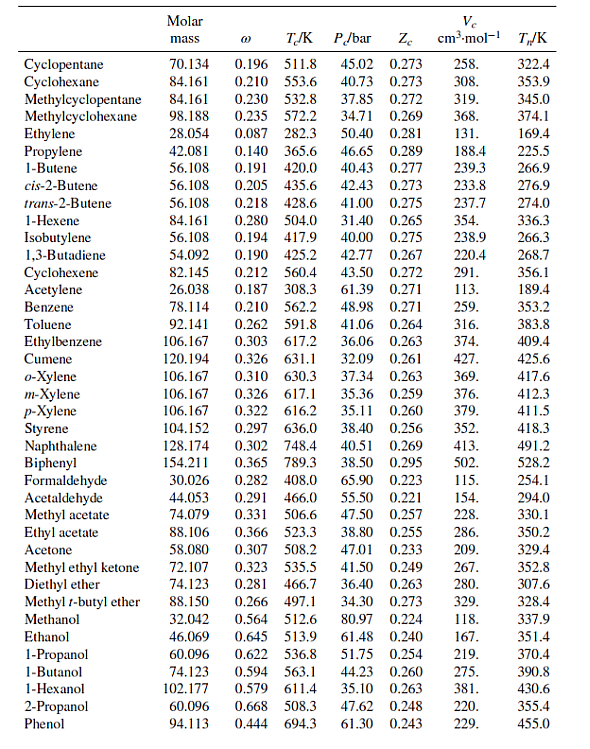
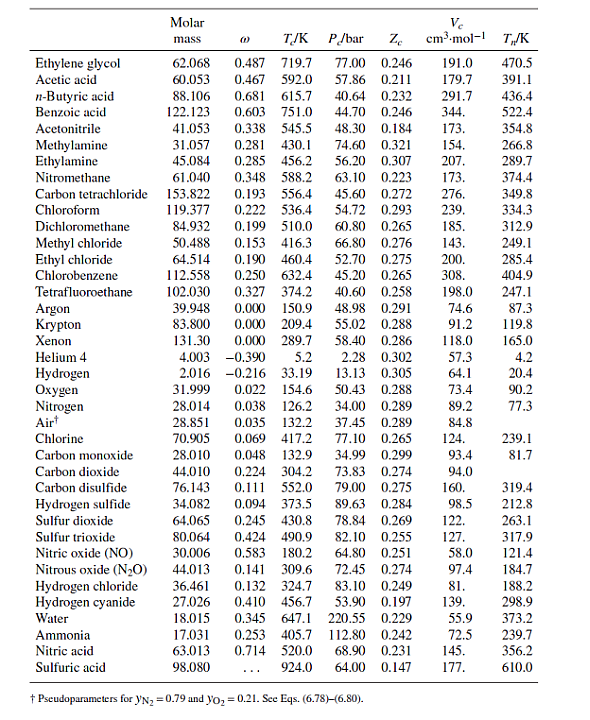
Methane Ethane Propane n-Butane n-Pentane n-Hexane n-Heptane n-Octane n-Nonane n-Decane Isobutane Isooctane Molar mass 16.043 30.070 44.097 58.123 72.150 86.177 100.204 114.231 128.258 142.285 58.123 114.231 @ TJK P/bar Ze 45.99 0.286 48.72 0.279 0.012 190.6 0.100 305.3 0.152 369.8 42.48 0.276 0.200 425.1 37.96 0.274 0.252 469.7 33.70 0.270 0.301 507.6 30.25 0.266 0.350 540.2 27.40 0.261 Ve cm.mol-1 98.6 145.5 200.0 255. 313. 371. 428. 0.400 568.7 24.90 0.256 0.444 594.6 22.90 0.252 0.492 617.7 21.10 0.247 0.181 408.1 36.48 0.282 262.7 0.302 544.0 25.68 0.266 468. 486. 544. 600. T/K 111.4 184.6 231.1 272.7 309.2 341.9 371.6 398.8 424.0 447.3 261.4 372.4 Cyclopentane Cyclohexane Methylcyclopentane Methylcyclohexane Ethylene Propylene 1-Butene cis-2-Butene trans-2-Butene 1-Hexene Isobutylene 1,3-Butadiene Cyclohexene Acetylene Benzene Toluene Ethylbenzene Cumene o-Xylene m-Xylene p-Xylene Styrene Naphthalene Biphenyl Formaldehyde Acetaldehyde Methyl acetate Ethyl acetate Acetone Methyl ethyl ketone Diethyl ether Methyl 1-butyl ether Methanol Ethanol 1-Propanol 1-Butanol 1-Hexanol 2-Propanol Phenol Molar mass TJK 0.196 511.8 0.210 553.6 70.134 84.161 84.161 0.230 532.8 98.188 0.235 572.2 28.054 0.087 282.3 42.081 0.140 365.6 56.108 0.191 420.0 56.108 0.205 435.6 88.106 58.080 P/bar Ze 45.02 0.273 40.73 0.273 37.85 0.272 56.108 0.218 428.6 41.00 0.275 84.161 0.280 504.0 31.40 0.265 56.108 40.00 0.275 0.194 417.9 0.190 425.2 54.092 42.77 0.267 82.145 0.212 560.4 43.50 0.272 26.038 0.187 308.3 61.39 0.271 78.114 0.210 562.2 48.98 0.271 0.262 591.8 41.06 0.264 92.141 106.167 0.303 617.2 36.06 0.263 120.194 0.326 631.1 32.09 0.261 37.34 0.263 106.167 0.310 630.3 106.167 0.326 617.1 35.36 0.259 106.167 0.322 616.2 35.11 0.260 104.152 38.40 0.256 0.297 636.0 0.302 748.4 128.174 40.51 0.269 154.211 0.365 789.3 38.50 0.295 30.026 0.282 408.0 0.291 466.0 44.053 74.079 0.331 506.6 0.366 523.3 0.307 508.2 0.323 535.5 0.281 466.7 0.266 497.1 72.107 74.123 88.150 32.042 0.564 512.6 46.069 0.645 513.9 60.096 0.622 536.8 74.123 102.177 34.71 0.269 50.40 0.281 46.65 0.289 40.43 0.277 42.43 0.273 Ve cm-mol-1 T/K 258. 308. 319. 368. 131. 188.4 239.3 233.8 237.7 354. 238.9 220.4 291. 113. 259. 316. 374. 427. 369. 376. 379. 352. 413. 502. 65.90 0.223 115. 55.50 0.221 154. 47.50 0.257 228. 38.80 0.255 286. 47.01 0.233 209. 41.50 0.249 267. 36.40 0.263 280. 34.30 0.273 329. 80.97 118. 167. 219. 0.224 61.48 0.240 51.75 0.254 0.594 563.1 44.23 0.260 275. 0.579 611.4 35.10 0.263 381. 0.668 508.3 47.62 0.248 220. 60.096 94.113 0.444 694.3 61.30 0.243 229. 322.4 353.9 345.0 374.1 169.4 225.5 266.9 276.9 274.0 336.3 266.3 268.7 356.1 189.4 353.2 383.8 409.4 425.6 417.6 412.3 411.5 418.3 491.2 528.2 254.1 294.0 330.1 350.2 329.4 352.8 307.6 328.4 337.9 351.4 370.4 390.8 430.6 355.4 455.0 Ethylene glycol Acetic acid n-Butyric acid Benzoic acid Acetonitrile Methylamine Ethylamine Nitromethane Carbon tetrachloride Chloroform Dichloromethane Methyl chloride Ethyl chloride Chlorobenzene Tetrafluoroethane Argon Krypton Xenon Helium 4 Hydrogen Oxygen Nitrogen Air Chlorine Carbon monoxide Carbon dioxide Carbon disulfide Hydrogen sulfide Sulfur dioxide Sulfur trioxide Nitric oxide (NO) Nitrous oxide (NO) Hydrogen chloride Hydrogen cyanide Water Molar mass Ammonia Nitric acid Sulfuric acid TJK P/bar Ze 62.068 0.487 719.7 77.00 0.246 60.053 0.467 592.0 88.106 0.681 615.7 122.123 0.603 751.0 0.338 545.5 41.053 31.057 84.932 50.488 64.514 112.558 102.030 (1) 0.281 430.1 45.084 0.285 456.2 61.040 0.348 588.2 153.822 0.193 556.4 45.60 119.377 0.222 536.4 0.199 510.0 0.153 416.3 39.948 83.800 131.30 57.86 0.211 179.7 40.64 0.232 291.7 28.014 28.851 0.035 132.2 70.905 0.069 417.2 28.010 0.048 132.9 44.010 0.224 304.2 76.143 0.111 552.0 34.082 89.63 0.284 78.84 0.269 82.10 0.255 0.094 373.5 64.065 0.245 430.8 80.064 0.424 490.9 30.006 0.583 44.013 0.141 309.6 36.461 0.132 324.7 0.410 456.7 53.90 0.197 180.2 64.80 0.251 72.45 0.274 83.10 0.249 27.026 18.015 0.345 647.1 220.55 0.229 0.253 405.7 112.80 0.242 17.031 63.013 0.714 520.0 68.90 0.231 98.080 924.0 64.00 0.147 Pseudoparameters for YN=0.79 and yo = 0.21. See Eqs. (6.78)-(6.80). 44.70 0.246 344. 48.30 0.184 173. 74.60 0.321 154. 56.20 0.307 207. 63.10 0.223 173. 0.272 54.72 0.293 60.80 0.265 66.80 0.276 0.190 460.4 52.70 0.275 0.250 632.4 45.20 0.327 374.2 40.60 0.258 0.000 150.9 48.98 0.291 0.000 209.4 55.02 0.288 0.000 289.7 58.40 0.286 4.003 -0.390 5.2 2.28 0.302 2.016 -0.216 33.19 13.13 0.305 31.999 0.022 154.6 50.43 0.288 0.038 126.2 34.00 0.289 Ve cm.mol-1 T/K 276. 239. 185. 143. 200. 0.265 308. 198.0 74.6 91.2 118.0 191.0 470.5 391.1 436.4 522.4 37.45 0.289 77.10 0.265 34.99 0.299 73.83 0.274 79.00 0.275 57.3 64.1 73.4 89.2 84.8 124. 93.4 94.0 160. 98.5 122. 127. 58.0 97.4 81. 139. 55.9 72.5 145. 177. 354.8 266.8 289.7 374.4 349.8 334.3 312.9 249.1 285.4 404.9 247.1 87.3 119.8 165.0 4.2 20.4 90.2 77.3 239.1 81.7 319.4 212.8 263.1 317.9 121.4 184.7 188.2 298.9 373.2 239.7 356.2 610.0
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


