Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Determine the equation of the best-fit line through your data for the plot of [KIO3] vs 1/t in the form y=mx+b. Using your

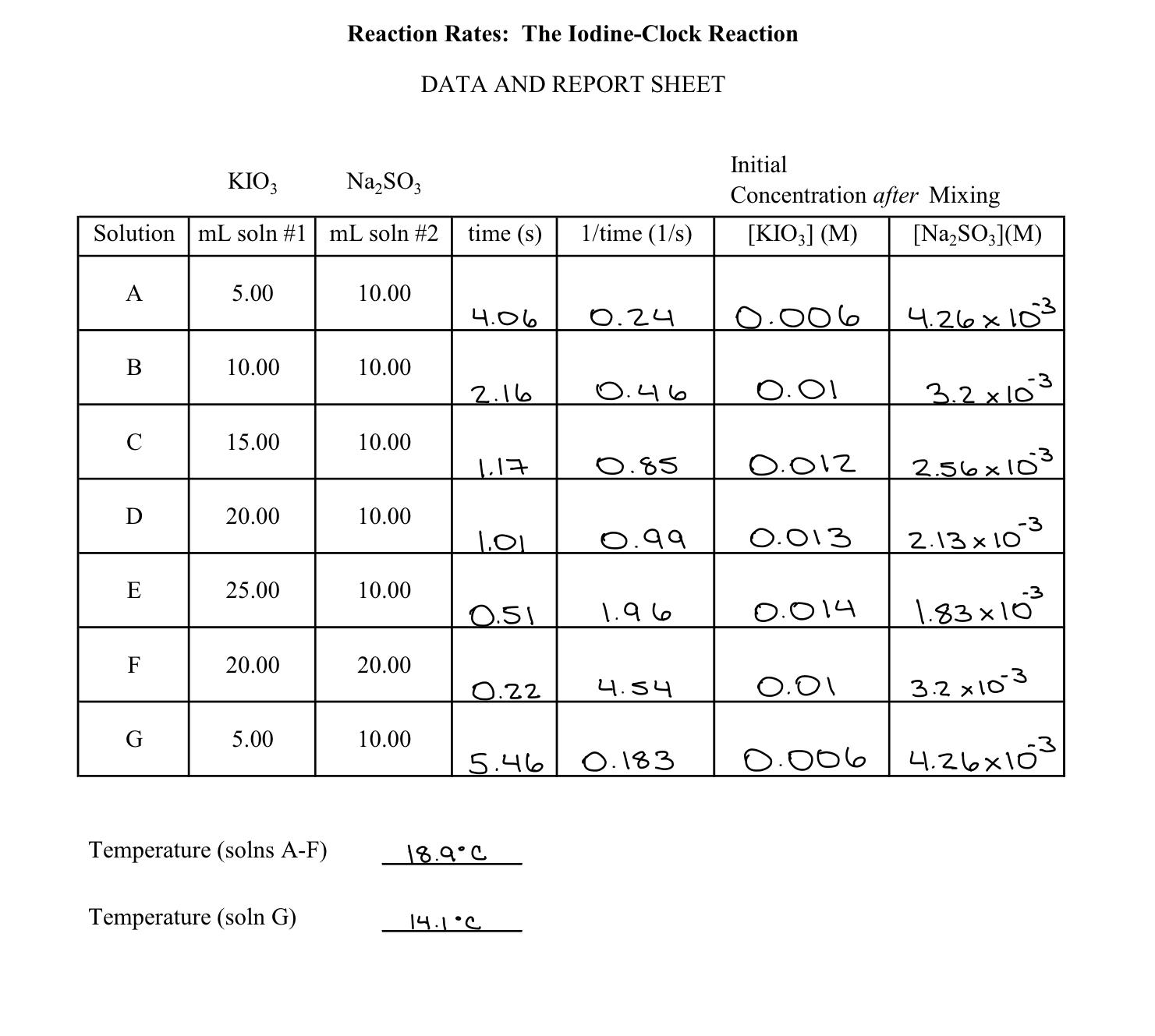
1. Determine the equation of the best-fit line through your data for the plot of [KIO3] vs 1/t in the form y=mx+b. Using your equation, fill in the following. Equation: a. m (slope) = b. b (y-intercept) = c. Using your equation, what is the expected inverse time at which [KIO3] = 1.5 x 103 M ? What is the expected reaction time interval? d. If the time for the reacton to occur is 3.5 minutes, what is the molar concentration of KIO3? Show calculations. 2. What can you conclude about the effect on the reaction time interval of increasing the concentration of sulfite ion while holding the concentration of iodate ion constant? Which two mixed solutions did you use to determine this? 3. What is the relationship of reaction time interval to temperature? Which two mixed solutions did you use to determine this? Reaction Rates: The Iodine-Clock Reaction DATA AND REPORT SHEET Initial KIO3 Na2SO3 Concentration after Mixing Solution mL soln #1 mL soln #2 time (s) 1/time (1/s) [KIO3] (M) [Na2SO3] (M) A 5.00 10.00 4.06 0.24 4.26x103 B 10.00 10.00 2.16 0.46 0.01 3.2 103 C 15.00 10.00 1.17 0.85 0.012 2.56103 D 20.00 10.00 1.01 0.99 0.013 2.13 103 [I] E 25.00 10.00 0.51 1.96 0.014 1.8310 F 20.00 20.00 0.22 4.54 0.01 3.2 103 G 5.00 10.00 5.46 0.183 0.006 4.26x103 Temperature (solns A-F) 18.9C Temperature (soln G) 14.1C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


