Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Tucked into Lecture 5 is an important result we now come back to. To recap: in order to calculate the average value of something
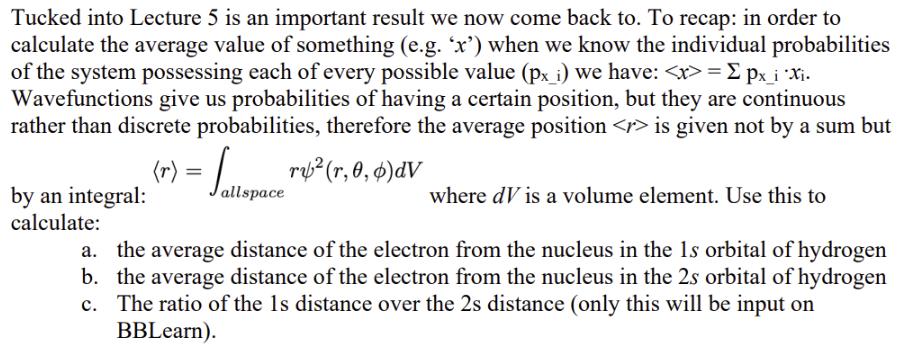
Tucked into Lecture 5 is an important result we now come back to. To recap: in order to calculate the average value of something (e.g. 'x') when we know the individual probabilities of the system possessing each of every possible value (px_i) we have: = px_i X. Wavefunctions give us probabilities of having a certain position, but they are continuous rather than discrete probabilities, therefore the average position is given not by a sum but (r) = Sallspace rub(r, 0, 6) av by an integral: calculate: where dV is a volume element. Use this to a. the average distance of the electron from the nucleus in the 1s orbital of hydrogen b. the average distance of the electron from the nucleus in the 2s orbital of hydrogen c. The ratio of the 1s distance over the 2s distance (only this will be input on BBLearn).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To calculate the average distance of the electron from the nucleus in the hydrogen atoms 1s and 2s o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


