Question
Two atoms, A and B, are indicated in the skeletal structure shown below. I am unsure what is wrong here, both of these have electron
Two atoms, A and B, are indicated in the skeletal structure shown below.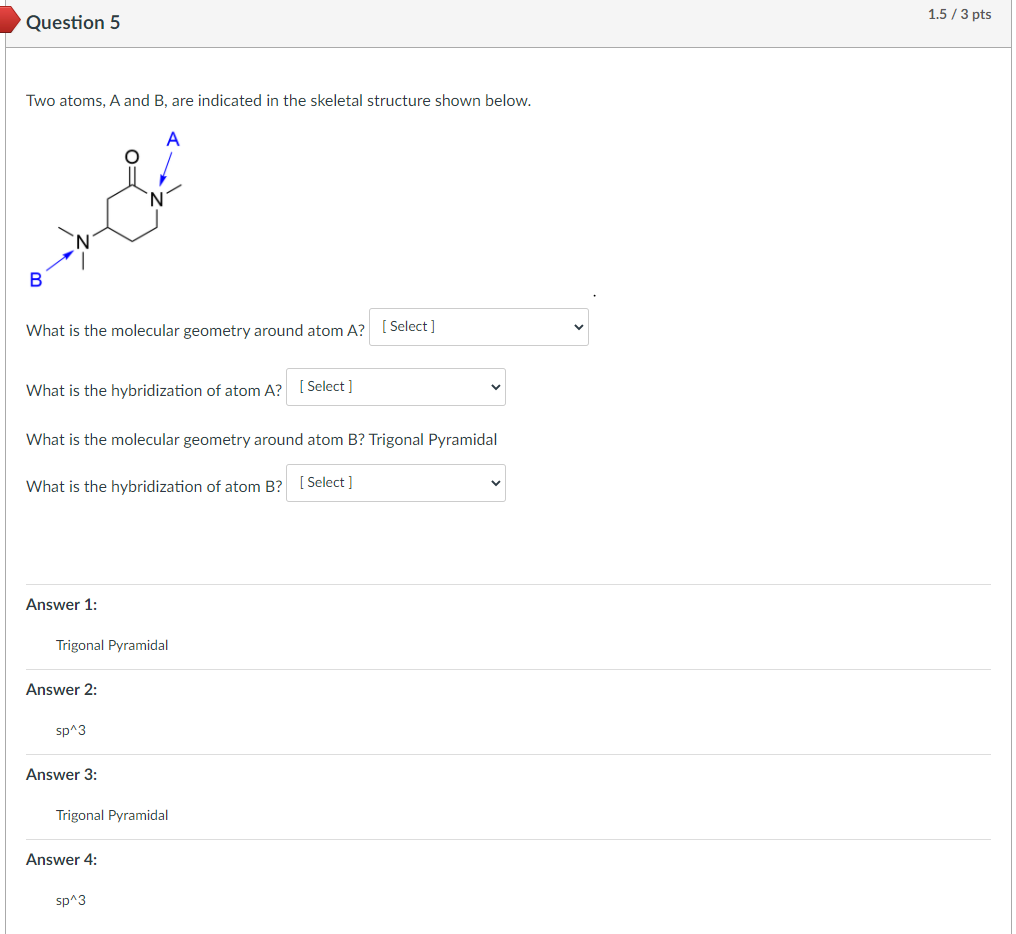
I am unsure what is wrong here, both of these have electron domains of 4. 3 bonds and 1 lone pair. So the hybridization should be sp^3 and the geometry should be trigonal pyramidal. I am unsure of what I am missing.
What is the molecular geometry around atom A? [ Select ] ["Trigonal Planar", "Linear", "Tetrahedral", "Trigonal Pyramidal", "Bent"]
What is the hybridization of atom A? [ Select ] ["sp^3", "sp", "sp^2"]
What is the molecular geometry around atom B? [ Select ] ["Trigonal Pyramidal", "Trigonal Planar", "Tetrahedral", "Bent", "Linear"]
What is the hybridization of atom B? sp^3
Question 5 1.5/3 pts Two atoms, A and B, are indicated in the skeletal structure shown below. B What is the molecular geometry around atom A? (Select] What is the hybridization of atom A? (Select] What is the molecular geometry around atom B? Trigonal Pyramidal What is the hybridization of atom B? [Select ] Answer 1: Trigonal Pyramidal Answer 2: sp^3 Answer 3: Trigonal Pyramidal Answer 4: sp^3Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


