Answered step by step
Verified Expert Solution
Question
1 Approved Answer
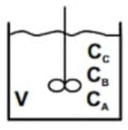
Two consecutive first-order reactions are carried out in a perfectly stirred, isothermal batch reactor. r a =k 1 Ca r b =k 2 Cb (Use
Two consecutive first-order reactions are carried out in a perfectly stirred, isothermal batch reactor.
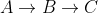
ra=k1Ca
rb=k2Cb
(Use Laplace transform for problem solving tool)
a) Considering constant density, obtain analytically the dynamic behavior of the concentrations of components A and B for the situation where k1 = k2. The concentration of A at the start of the batch is Ca0. Initially there are no B and C components in the reactor.
b) What will be the maximum concentration of component B produced and when does this occur?
A HB - Cc do |V CAStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


