Answered step by step
Verified Expert Solution
Question
1 Approved Answer
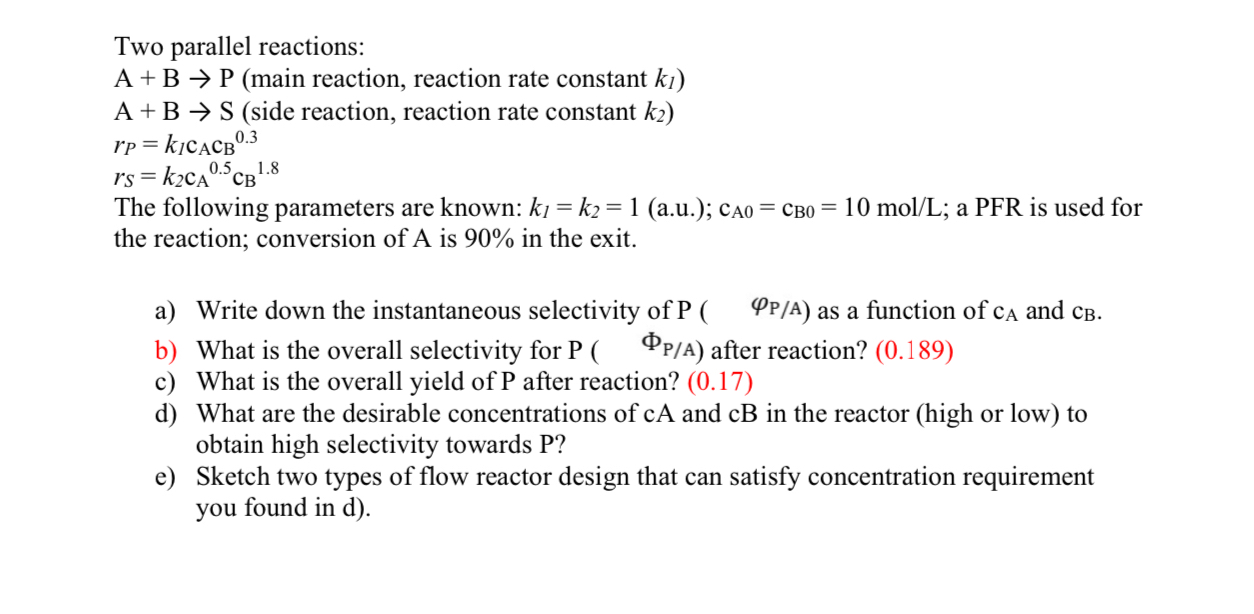
Two parallel reactions: A + B P ( main reaction, reaction rate constant k 1 ) A + B S ( side reaction, reaction rate
Two parallel reactions:
main reaction, reaction rate constant
side reaction, reaction rate constant
The following parameters are known: au; ; a PFR is used for the reaction; conversion of is in the exit.
a Write down the instantaneous selectivity of as a function of and
b What is the overall selectivity for P after reaction?
c What is the overall yield of after reaction?
d What are the desirable concentrations of and in the reactor high or low to obtain high selectivity towards P
e Sketch two types of flow reactor design that can satisfy concentration requirement you found in d
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


