Question
Two portions of 50.0 g of water, at 13.0 and 54.0 C, respectively, were mixed inside a cup calorimeter. If the temperature of water
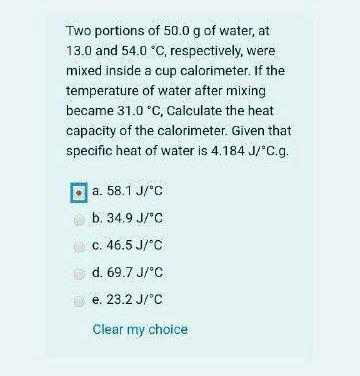
Two portions of 50.0 g of water, at 13.0 and 54.0 C, respectively, were mixed inside a cup calorimeter. If the temperature of water after mixing became 31.0 *C, Calculate the heat capacity of the calorimeter. Given that specific heat of water is 4.184 J/'C.g. a. 58.1 J/C b. 34.9 J/C c. 46.5 J/"C d. 69.7 J/C e. 23.2 J/"C Clear my choice
Step by Step Solution
3.49 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Chemical Engineering Thermodynamics
Authors: J. M. Smith, H. C. Van Ness, M. M. Abbott
7th edition
71247084, 978-0071247085
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



