Answered step by step
Verified Expert Solution
Question
1 Approved Answer
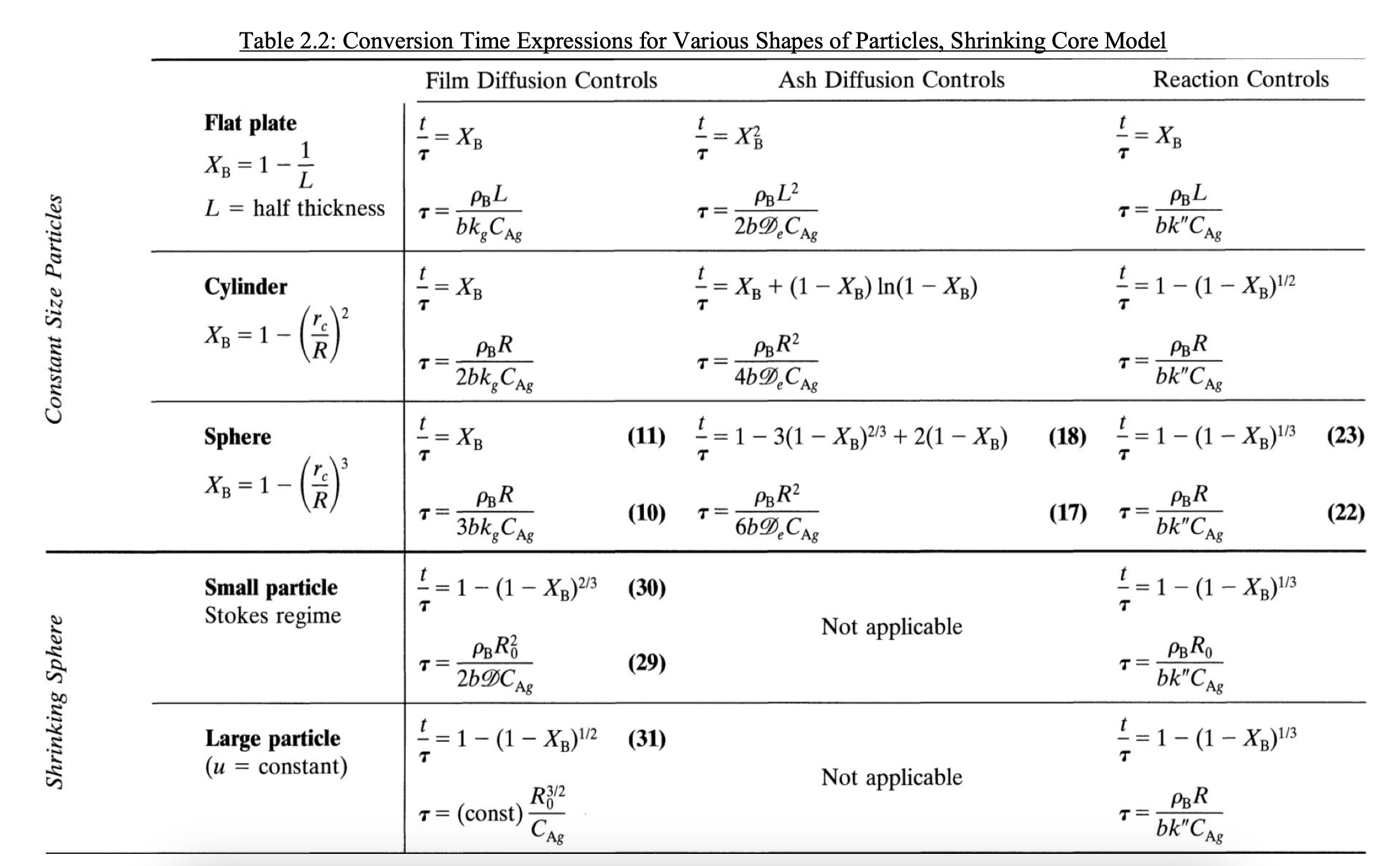
Two solid samples are introduced into a constant medium oven and left for 1 hour. Under these conditions, particles with an initial radius of 4
Two solid samples are introduced into a constant medium oven and left for hour. Under these conditions, particles with an initial radius of mm undergo conversion, and particles of mm convert at
a Find the mechanism controlling the reaction rate of this solid, considering that the size of the particles does not change over time.
b Find the time required for complete conversion of particles with a radius of mm
Please consider the resolution by these relations Image I saw many resolutions that did not coherently explain the resolution by this. One resolution shows that the control is by reaction but calculates just using de Xb conversion of why dont use de other conversion or a relation betwenn then?
Thank you
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


