Two types of singly ionized atoms have the same charge q, but whose masses differ by only a small amount Am, are introduced into
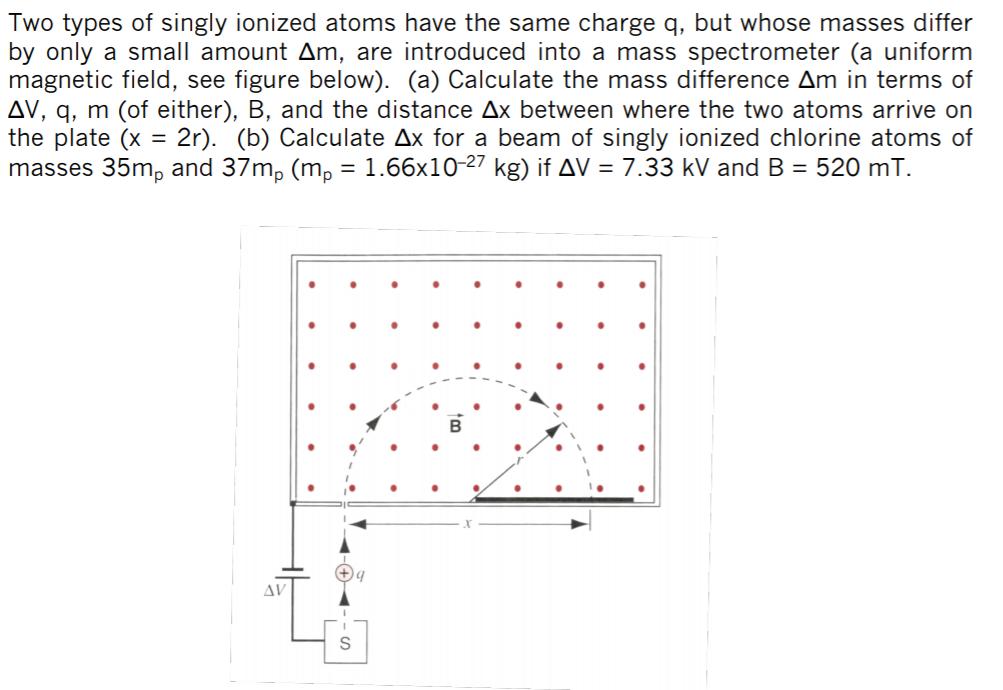
Two types of singly ionized atoms have the same charge q, but whose masses differ by only a small amount Am, are introduced into a mass spectrometer (a uniform magnetic field, see figure below). (a) Calculate the mass difference Am in terms of AV, q, m (of either), B, and the distance Ax between where the two atoms arrive on the plate (x 2r). (b) Calculate Ax for a beam of singly ionized chlorine atoms of masses 35m, and 37m, (m, = 1.66x10-27 kg) if AV = 7.33 kV and B = 520 mT. AV
Step by Step Solution
3.54 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Kinelte enengy K oV Radtus of cuvahone ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


