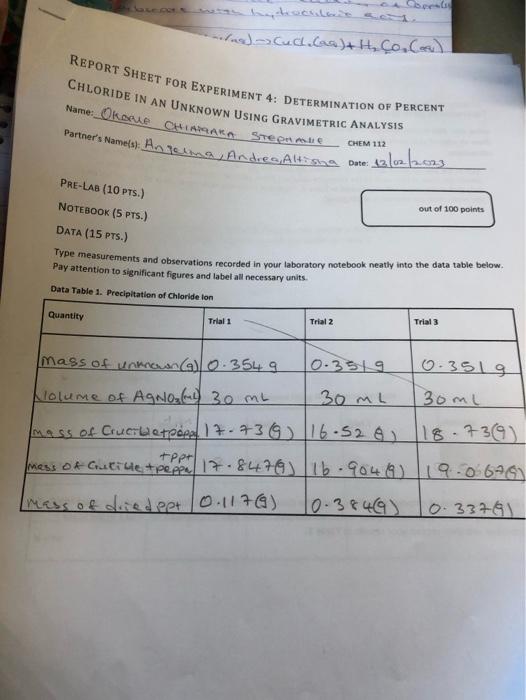
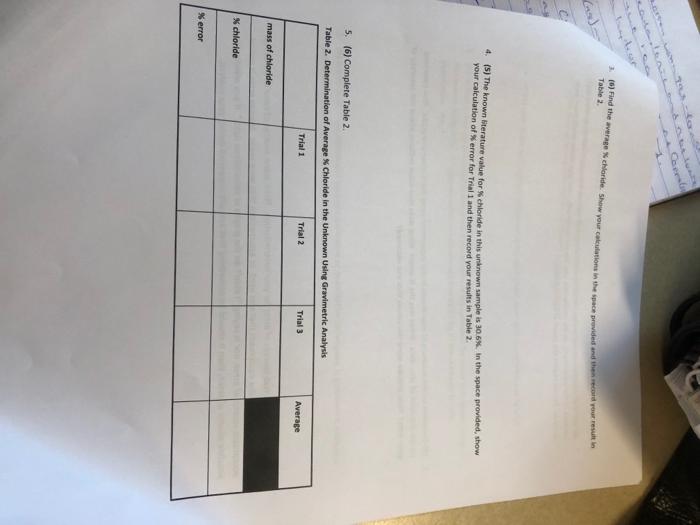
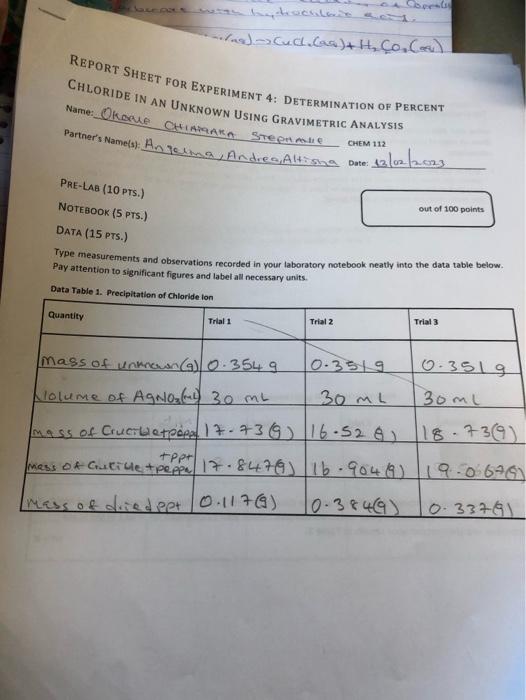
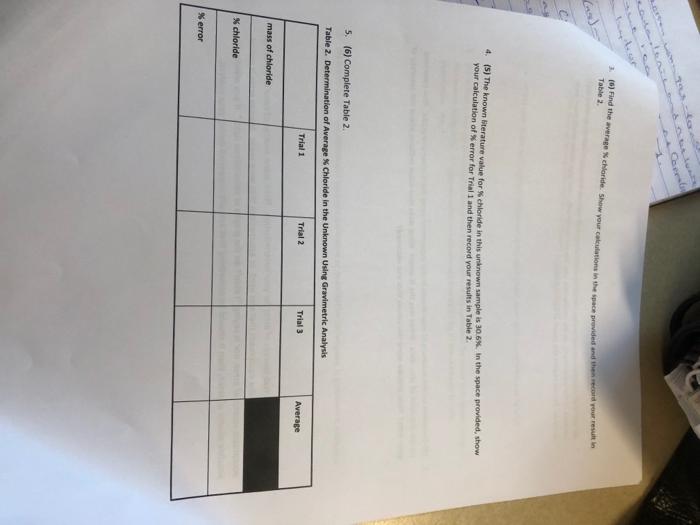
Type measurements and observations recorded in your laboratory notebook neatly into the data table below. Pay attention to significant figures and label all necessary units. POST-LA0 QuIstions (15 prsi) 1. (4) Answer the following cututions regarding the physical propertiei of the twe spidid obrened in this tab. a. What color is the original ininown compound in this experiment? b. What color is the ABCl precipitate formed in this wxperiment upon addition of HNON and AgNO2 ? 2. (5) in Step 1, you are instructed to weigh out three samples of the unknown "to about 0.35 grams (weigh to the nearest miligram), It is not necessary to measure out exactly 0.35g of the unknown for this experiment. Why? 3. (6) Briefly outline the differences between qualifatiye ar quantitathe techniques. Is gravimetric analysis a qualifative ar quanititathe technigue? Explain your choice: 1. (3) In 1-2 sentences, summanie vour most ingartant resuit and indicate how your findings tuifuled the Aurpose of the experimient. In the conclutlon, you should repart only your one major result trom your enalyuis. Do not report the result at each sub-step. 2. (3) Comment on the accuracy of your results in this experiment using the Nerror as a guide. Also comment. on the precision of your data. What was the K error? What does that value indicate abeut the accuracy of your data? How precise were the values that you obtained? 3. (4) Sugest at least two sources of error for your determination. Think about sources of error in the design of the experiment, the equipment that was used, or assumptions that were made. Human error is not an. acceptable source of ermor, nor is saying "I didn't do the graphs or calculations right." If you mention a possible source of error, then it should be something that actually happened during yout analysis. 3. (6) Find the average K chloride. Show your colrulations in the space privided and then recoid raur resuit in Table 2. 4. (5) The known titerature value for % chloride in this unknown sample is 30.6. in the space nrovided, show your caiculation of $ error for Trial 1 and then record vour resufts in Table 2. 5. (6) Complete Table 2 . Table 2. Determination of Averake S Chloride in the Uniknown Usine Gravimatrie Analuele: TAEATMENT OF DATA ( 45 PTS.) USing your data and information in the General Chemisery 1 Loboratory Monual, calculate the following valuen. Show all steps of vour work, be sure to use the proper number of significant figures and include all units in your calculations. 1. (22) in lab, you obtained the mass of the dried AgCl precipitate. Now, use stolchiometry calculations to obtain the mass of Cl in each of the three precipitates. Show your calculations for Trial 1 in the space provided and then record your results in Table 2. Note that the molar moss for silver is 107.87g/mol and the molar mass for chlorine is 35.45g/hnd. 2. (6) Using the mass of chlorine from Step 1 and the data from Data Table 1 for each sample, calculate the \% chloride by mass in each of the three unknown samples. Show your calculations for Trial 1 in the 5pace provided and then record your results in Table 2. Type measurements and observations recorded in your laboratory notebook neatly into the data table below. Pay attention to significant figures and label all necessary units. TAEATMENT OF DATA ( 45 PTS.) USing your data and information in the General Chemisery 1 Loboratory Monual, calculate the following valuen. Show all steps of vour work, be sure to use the proper number of significant figures and include all units in your calculations. 1. (22) in lab, you obtained the mass of the dried AgCl precipitate. Now, use stolchiometry calculations to obtain the mass of Cl in each of the three precipitates. Show your calculations for Trial 1 in the space provided and then record your results in Table 2. Note that the molar moss for silver is 107.87g/mol and the molar mass for chlorine is 35.45g/hnd. 2. (6) Using the mass of chlorine from Step 1 and the data from Data Table 1 for each sample, calculate the \% chloride by mass in each of the three unknown samples. Show your calculations for Trial 1 in the 5pace provided and then record your results in Table 2. 3. (6) Find the average K chloride. Show your colrulations in the space privided and then recoid raur resuit in Table 2. 4. (5) The known titerature value for % chloride in this unknown sample is 30.6. in the space nrovided, show your caiculation of $ error for Trial 1 and then record vour resufts in Table 2. 5. (6) Complete Table 2 . Table 2. Determination of Averake S Chloride in the Uniknown Usine Gravimatrie Analuele: 1. (3) In 1-2 sentences, summanie vour most ingartant resuit and indicate how your findings tuifuled the Aurpose of the experimient. In the conclutlon, you should repart only your one major result trom your enalyuis. Do not report the result at each sub-step. 2. (3) Comment on the accuracy of your results in this experiment using the Nerror as a guide. Also comment. on the precision of your data. What was the K error? What does that value indicate abeut the accuracy of your data? How precise were the values that you obtained? 3. (4) Sugest at least two sources of error for your determination. Think about sources of error in the design of the experiment, the equipment that was used, or assumptions that were made. Human error is not an. acceptable source of ermor, nor is saying "I didn't do the graphs or calculations right." If you mention a possible source of error, then it should be something that actually happened during yout analysis. POST-LA0 QuIstions (15 prsi) 1. (4) Answer the following cututions regarding the physical propertiei of the twe spidid obrened in this tab. a. What color is the original ininown compound in this experiment? b. What color is the ABCl precipitate formed in this wxperiment upon addition of HNON and AgNO2 ? 2. (5) in Step 1, you are instructed to weigh out three samples of the unknown "to about 0.35 grams (weigh to the nearest miligram), It is not necessary to measure out exactly 0.35g of the unknown for this experiment. Why? 3. (6) Briefly outline the differences between qualifatiye ar quantitathe techniques. Is gravimetric analysis a qualifative ar quanititathe technigue? Explain your choice: Type measurements and observations recorded in your laboratory notebook neatly into the data table below. Pay attention to significant figures and label all necessary units. POST-LA0 QuIstions (15 prsi) 1. (4) Answer the following cututions regarding the physical propertiei of the twe spidid obrened in this tab. a. What color is the original ininown compound in this experiment? b. What color is the ABCl precipitate formed in this wxperiment upon addition of HNON and AgNO2 ? 2. (5) in Step 1, you are instructed to weigh out three samples of the unknown "to about 0.35 grams (weigh to the nearest miligram), It is not necessary to measure out exactly 0.35g of the unknown for this experiment. Why? 3. (6) Briefly outline the differences between qualifatiye ar quantitathe techniques. Is gravimetric analysis a qualifative ar quanititathe technigue? Explain your choice: 1. (3) In 1-2 sentences, summanie vour most ingartant resuit and indicate how your findings tuifuled the Aurpose of the experimient. In the conclutlon, you should repart only your one major result trom your enalyuis. Do not report the result at each sub-step. 2. (3) Comment on the accuracy of your results in this experiment using the Nerror as a guide. Also comment. on the precision of your data. What was the K error? What does that value indicate abeut the accuracy of your data? How precise were the values that you obtained? 3. (4) Sugest at least two sources of error for your determination. Think about sources of error in the design of the experiment, the equipment that was used, or assumptions that were made. Human error is not an. acceptable source of ermor, nor is saying "I didn't do the graphs or calculations right." If you mention a possible source of error, then it should be something that actually happened during yout analysis. 3. (6) Find the average K chloride. Show your colrulations in the space privided and then recoid raur resuit in Table 2. 4. (5) The known titerature value for % chloride in this unknown sample is 30.6. in the space nrovided, show your caiculation of $ error for Trial 1 and then record vour resufts in Table 2. 5. (6) Complete Table 2 . Table 2. Determination of Averake S Chloride in the Uniknown Usine Gravimatrie Analuele: TAEATMENT OF DATA ( 45 PTS.) USing your data and information in the General Chemisery 1 Loboratory Monual, calculate the following valuen. Show all steps of vour work, be sure to use the proper number of significant figures and include all units in your calculations. 1. (22) in lab, you obtained the mass of the dried AgCl precipitate. Now, use stolchiometry calculations to obtain the mass of Cl in each of the three precipitates. Show your calculations for Trial 1 in the space provided and then record your results in Table 2. Note that the molar moss for silver is 107.87g/mol and the molar mass for chlorine is 35.45g/hnd. 2. (6) Using the mass of chlorine from Step 1 and the data from Data Table 1 for each sample, calculate the \% chloride by mass in each of the three unknown samples. Show your calculations for Trial 1 in the 5pace provided and then record your results in Table 2. Type measurements and observations recorded in your laboratory notebook neatly into the data table below. Pay attention to significant figures and label all necessary units. TAEATMENT OF DATA ( 45 PTS.) USing your data and information in the General Chemisery 1 Loboratory Monual, calculate the following valuen. Show all steps of vour work, be sure to use the proper number of significant figures and include all units in your calculations. 1. (22) in lab, you obtained the mass of the dried AgCl precipitate. Now, use stolchiometry calculations to obtain the mass of Cl in each of the three precipitates. Show your calculations for Trial 1 in the space provided and then record your results in Table 2. Note that the molar moss for silver is 107.87g/mol and the molar mass for chlorine is 35.45g/hnd. 2. (6) Using the mass of chlorine from Step 1 and the data from Data Table 1 for each sample, calculate the \% chloride by mass in each of the three unknown samples. Show your calculations for Trial 1 in the 5pace provided and then record your results in Table 2. 3. (6) Find the average K chloride. Show your colrulations in the space privided and then recoid raur resuit in Table 2. 4. (5) The known titerature value for % chloride in this unknown sample is 30.6. in the space nrovided, show your caiculation of $ error for Trial 1 and then record vour resufts in Table 2. 5. (6) Complete Table 2 . Table 2. Determination of Averake S Chloride in the Uniknown Usine Gravimatrie Analuele: 1. (3) In 1-2 sentences, summanie vour most ingartant resuit and indicate how your findings tuifuled the Aurpose of the experimient. In the conclutlon, you should repart only your one major result trom your enalyuis. Do not report the result at each sub-step. 2. (3) Comment on the accuracy of your results in this experiment using the Nerror as a guide. Also comment. on the precision of your data. What was the K error? What does that value indicate abeut the accuracy of your data? How precise were the values that you obtained? 3. (4) Sugest at least two sources of error for your determination. Think about sources of error in the design of the experiment, the equipment that was used, or assumptions that were made. Human error is not an. acceptable source of ermor, nor is saying "I didn't do the graphs or calculations right." If you mention a possible source of error, then it should be something that actually happened during yout analysis. POST-LA0 QuIstions (15 prsi) 1. (4) Answer the following cututions regarding the physical propertiei of the twe spidid obrened in this tab. a. What color is the original ininown compound in this experiment? b. What color is the ABCl precipitate formed in this wxperiment upon addition of HNON and AgNO2 ? 2. (5) in Step 1, you are instructed to weigh out three samples of the unknown "to about 0.35 grams (weigh to the nearest miligram), It is not necessary to measure out exactly 0.35g of the unknown for this experiment. Why? 3. (6) Briefly outline the differences between qualifatiye ar quantitathe techniques. Is gravimetric analysis a qualifative ar quanititathe technigue? Explain your choice