Answered step by step
Verified Expert Solution
Question
1 Approved Answer
U - Chapter 7: Homework 1 Chapter 7: Homework 1 Question 7 of 8 Chapter 7: Homework 1 Question 7 of 8 Part 3 (c)
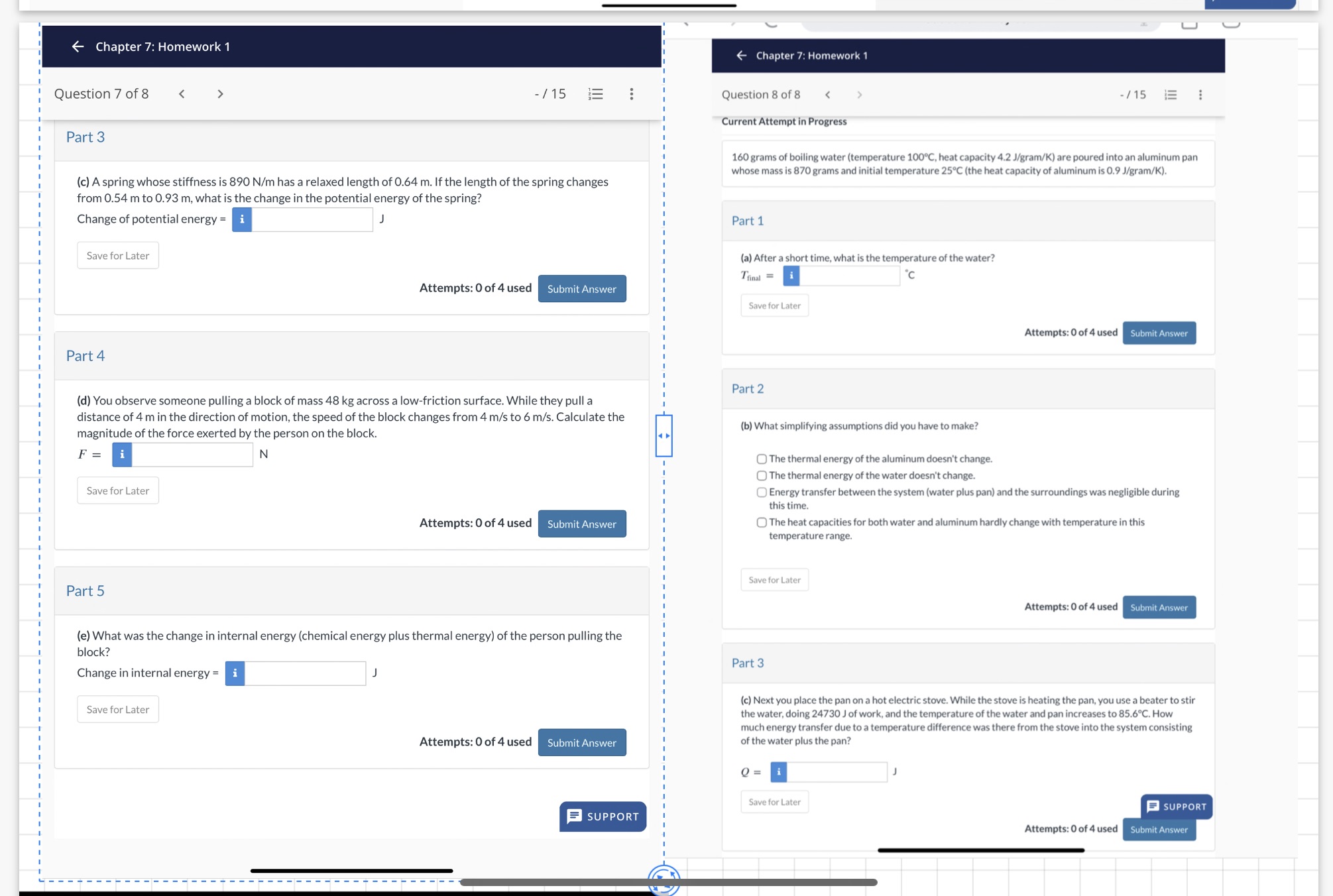
Chapter 7: Homework 1 Question 7 of 8 Part 3 (c) A spring whose stiffness is 890 N/m has a relaxed length Of 0.64 m. If the length Of the spring changes from 0.54 m to 0.93 m, what is the change in the potential energy of the spring? Chapter 7: Homework 1 Question 8 of 8 Current Attempt in Progress 160 grams Of boiling water (temperature , heat capacity 4.2 J/gram/K) are poured into an aluminum pan whose mass is 870 grams and initial temperature 25C (the heat capacity of aluminum is 0.9 J/gram/K). Change Of potential energy = Save for Later Part 4 Attempts: O Of 4 used Submit Answer Part 1 (a) After a short time. what is the temperature Of the water? c Save for Later Attempts: O of 4 used Part 2 (b) What simplifying assumptions did you have to make? C) The thermal energy Of the doesn't change. C) The thermal energy Of the water doesn't change. Submit Answer (d) You observe someone pulling a block of mass 48 kg across a low-friction surface. While they pull a distance of 4 m in the direction of motion, the speed of the block changes from 4 m/s to 6 m/s. Calculate the magnitude of the force exerted by the person on the block. Save for Later Attempts: O of 4 used Part 5 Submit Answer C) Energy transfer between the system (water plus pan) and the surroundings was negligible during this time. C) The heat capacities for both water and aluminum hardly change with temperature in this temperature range. Save for Later Attempts: O of 4 used Part 3 Submit Answer (e) What was the change in internal energy (chemical energy plus thermal energy) of the person pulling the block? Change in internal energy = Save for Later (c) Next you place the pan on a hot electric stove. While the stove is heating the pan. you use a beater to stir the water, doing 24730 J Of work, and the temperature Of the water and pan increases to 85.60C. How much energy transfer due to a temperature difference was there from the stove into the system consisting Attempts: O of 4 used Submit Answer SUPPORT of the water plus the pan? Save for Later Attempts: O of 4 used SUPPORT Submit Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


