Answered step by step
Verified Expert Solution
Question
1 Approved Answer
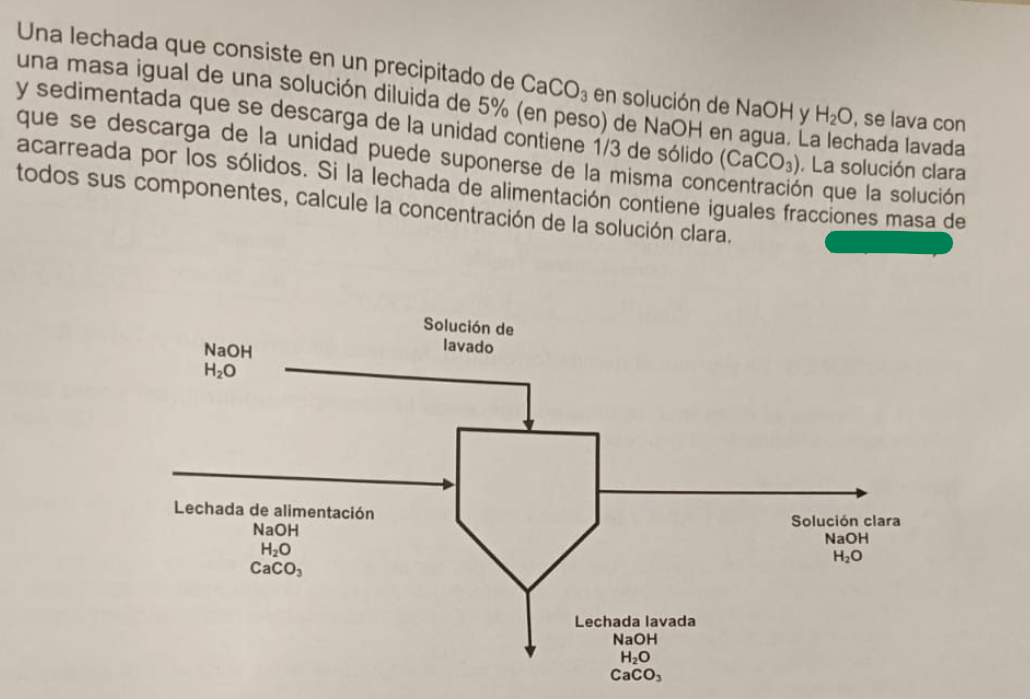
Una lechada que consiste en un precipitado de C a C O 3 en soluci n de N a O H 2 H 2 O
Una lechada que consiste en un precipitado de en solucin de se lava con una masa igual de una solucin diluida de en peso de NaOH en agua. La lechada lavada y sedimentada que se descarga de la unidad contiene de slido La solucin clara que se descarga de la unidad puede suponerse de la misma concentracin que la solucin acarreada por los slidos Si la lechada de alimentacin contiene iguales fracciones masa de todos sus componentes, calcule la concentracin de la solucin clara.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


