Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Under a constant pressure of 1.000atm, we do the combustion of 1.0000mol of propane, C3H8(g), at 25.0C (N.B. combustion is the reaction of a substance
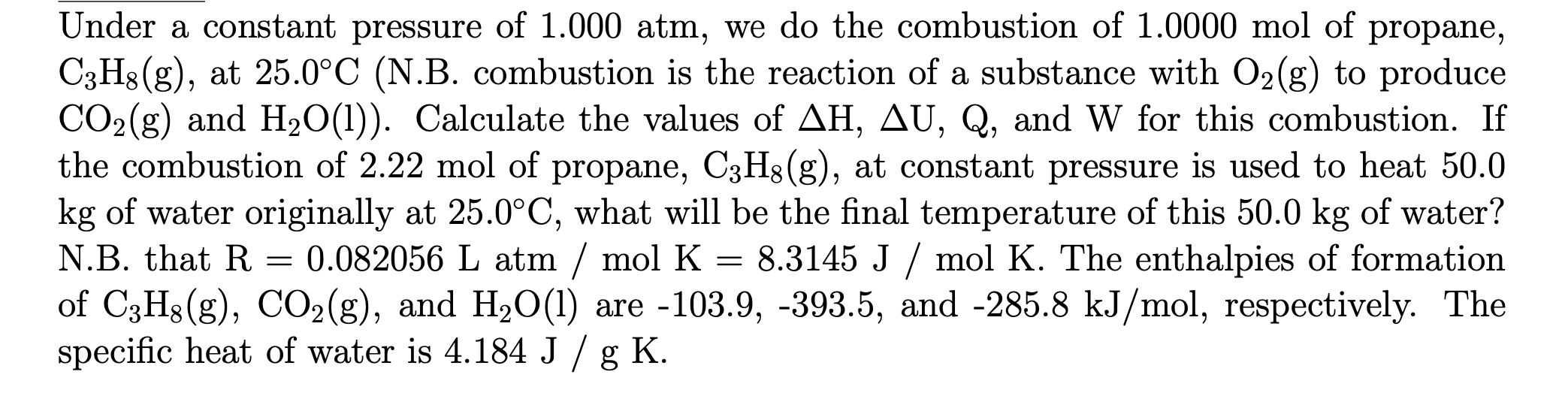 Under a constant pressure of 1.000atm, we do the combustion of 1.0000mol of propane, C3H8(g), at 25.0C (N.B. combustion is the reaction of a substance with O2(g) to produce CO2(g) and H2O(l)). Calculate the values of H,U,Q, and W for this combustion. If the combustion of 2.22mol of propane, C3H8(g), at constant pressure is used to heat 50.0 kg of water originally at 25.0C, what will be the final temperature of this 50.0kg of water? N.B. that R=0.082056L atm / mol K =8.3145J/molK. The enthalpies of formation of C3H8(g),CO2(g), and H2O(l) are 103.9,393.5, and 285.8kJ/mol, respectively. The specific heat of water is 4.184J/gK
Under a constant pressure of 1.000atm, we do the combustion of 1.0000mol of propane, C3H8(g), at 25.0C (N.B. combustion is the reaction of a substance with O2(g) to produce CO2(g) and H2O(l)). Calculate the values of H,U,Q, and W for this combustion. If the combustion of 2.22mol of propane, C3H8(g), at constant pressure is used to heat 50.0 kg of water originally at 25.0C, what will be the final temperature of this 50.0kg of water? N.B. that R=0.082056L atm / mol K =8.3145J/molK. The enthalpies of formation of C3H8(g),CO2(g), and H2O(l) are 103.9,393.5, and 285.8kJ/mol, respectively. The specific heat of water is 4.184J/gK Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


