Uniformity of Dosage units Consider a 300mg Rifampin capsule where 300mg Rifampin active plus 25 mg of filler/inert material and a 15mg hard capsule shell. Work out Table 1 under to determine whether CU or WV should be used, then refer to the Rifampin monograph to compare what is written. Speculate why USP chose the method they did.
I attached a snippet of Table 1 and the Rifampin monograph for reference.
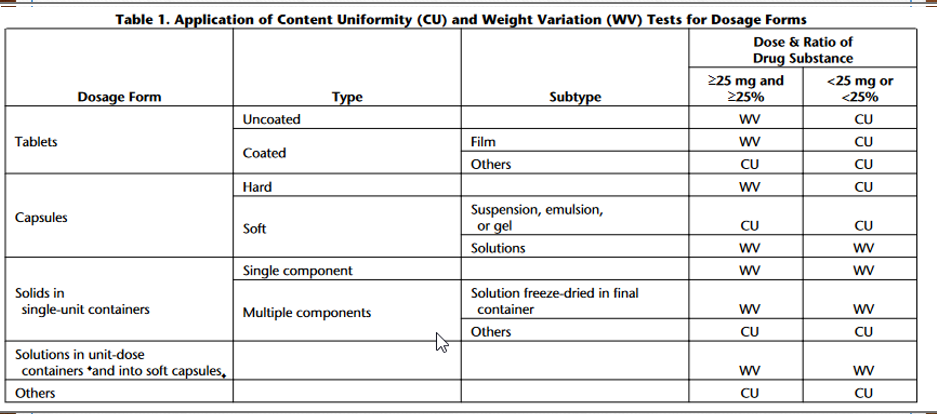
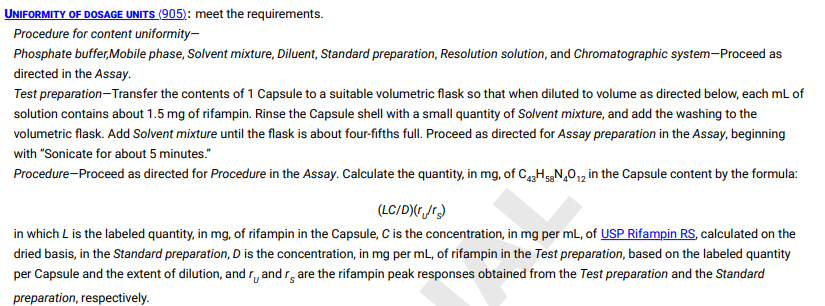
Table 1. Application of Content Uniformity (CU) and Weight Variation (WV) Tests for Dosage Forms UNIFORMITY OF DOSAGE UNITS (905) : meet the requirements. Procedure for content uniformity- Phosphate buffer,Mobile phase, Solvent mixture, Diluent, Standard preparation, Resolution solution, and Chromatographic system-Proceed as directed in the Assay. Test preparation-Transfer the contents of 1 Capsule to a suitable volumetric flask so that when diluted to volume as directed below, each mL of solution contains about 1.5mg of rifampin. Rinse the Capsule shell with a small quantity of Solvent mixture, and add the washing to the volumetric flask. Add Solvent mixture until the flask is about four-fifths full. Proceed as directed for Assay preparation in the Assay, beginning with "Sonicate for about 5 minutes." Procedure-Proceed as directed for Procedure in the Assay. Calculate the quantity, in mg, of C43H58N4O12 in the Capsule content by the formula: (LC/D)(ru/rS) in which L is the labeled quantity, in mg, of rifampin in the Capsule, C is the concentration, in mg per mL, of dried basis, in the Standard preparation, D is the concentration, in mg per mL, of rifampin in the Test preparation, based on the labeled quantity per Capsule and the extent of dilution, and rU and rS are the rifampin peak responses obtained from the Test preparation and the Standard preparation, respectively. Table 1. Application of Content Uniformity (CU) and Weight Variation (WV) Tests for Dosage Forms UNIFORMITY OF DOSAGE UNITS (905) : meet the requirements. Procedure for content uniformity- Phosphate buffer,Mobile phase, Solvent mixture, Diluent, Standard preparation, Resolution solution, and Chromatographic system-Proceed as directed in the Assay. Test preparation-Transfer the contents of 1 Capsule to a suitable volumetric flask so that when diluted to volume as directed below, each mL of solution contains about 1.5mg of rifampin. Rinse the Capsule shell with a small quantity of Solvent mixture, and add the washing to the volumetric flask. Add Solvent mixture until the flask is about four-fifths full. Proceed as directed for Assay preparation in the Assay, beginning with "Sonicate for about 5 minutes." Procedure-Proceed as directed for Procedure in the Assay. Calculate the quantity, in mg, of C43H58N4O12 in the Capsule content by the formula: (LC/D)(ru/rS) in which L is the labeled quantity, in mg, of rifampin in the Capsule, C is the concentration, in mg per mL, of dried basis, in the Standard preparation, D is the concentration, in mg per mL, of rifampin in the Test preparation, based on the labeled quantity per Capsule and the extent of dilution, and rU and rS are the rifampin peak responses obtained from the Test preparation and the Standard preparation, respectively








