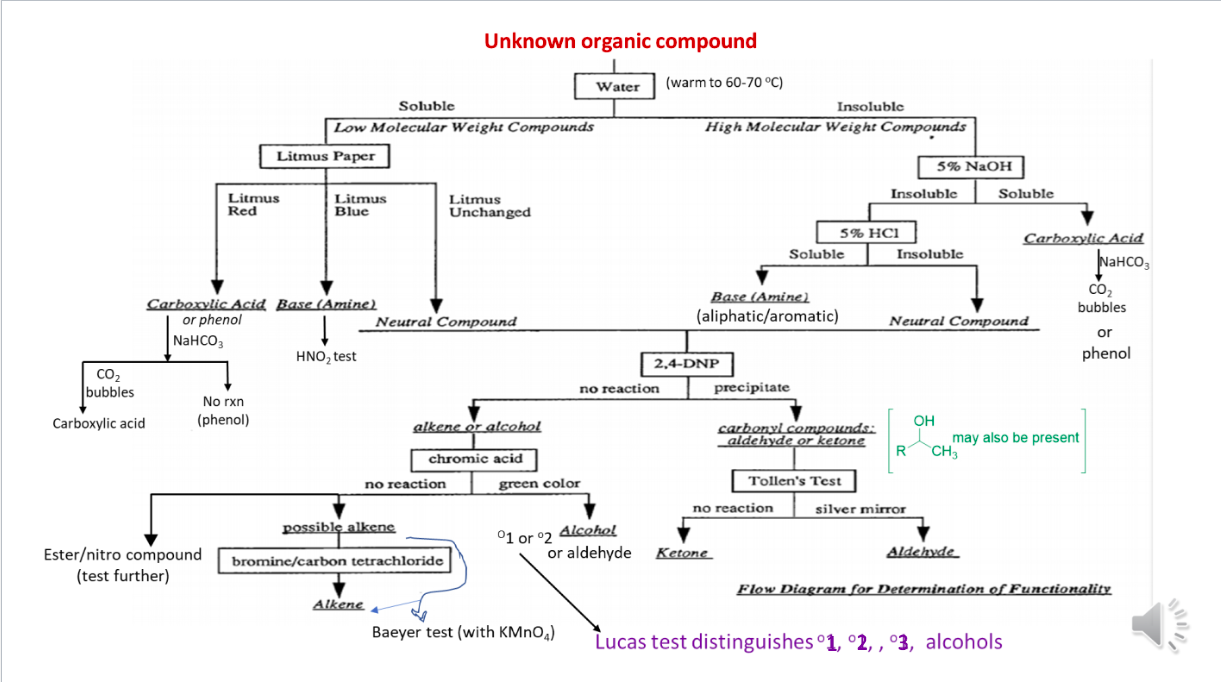
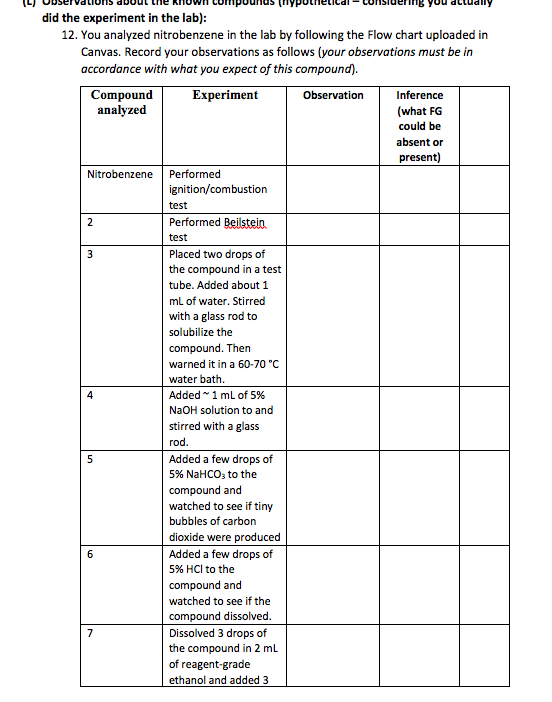
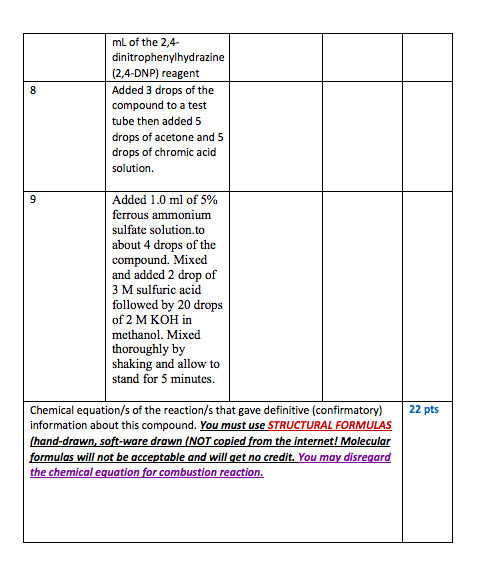
Unknown organic compound Water Soluble Low Molecular Weight Compounds Litmus Paper (warm to 60-70 C) Insoluble High Molecular Weight Compounds 5% NaOH Insoluble Soluble Litmus Red Litmus Blue Litmus Unchanged Carboxylic Acid Base (Amine) or phenol Neutral Compound NaHCO3 HNO, test CO2 bubbles No rxn Carboxylic acid (phenol) alkene or alcohol 5% HCI Carboxylic Acid Soluble Insoluble Naco, Base (Amine) CO2 (aliphatic/aromatic) bubbles Neutral Compound or 2,4-DNP phenol no reaction precipitate carbonyl compounds: aldehyde or ketone OH may also be present R CH3 chromic acid no reaction green color Tollen's Test no reaction silver mirror possible alkene 1 or 2 Alcoho! or aldehyde bromine/carbon tetrachloride Ketone Aldehyde Esteritro compound (test further) Flow Diagram for Determination of Functionality Alkene Baeyer test (with KMnO4) Lucas test distinguishes 1, 2, , '3, alcohols did the experiment in the lab): 12. You analyzed nitrobenzene in the lab by following the Flow chart uploaded in Canvas. Record your observations as follows (your observations must be in accordance with what you expect of this compound). Compound Experiment Observation Inference analyzed (what FG could be absent or present) Nitrobenzene Performed ignition/combustion test 2 Performed Beilstein test 3 Placed two drops of the compound in a test tube. Added about 1 mL of water. Stirred with a glass rod to solubilize the compound. Then warned it in a 60-70 C water bath. Added 1 mL of 5% NaOH solution to and stirred with a glass rod. Added a few drops of 5% NaHCO3 to the compound and watched to see if tiny bubbles of carbon dioxide were produced Added a few drops of 5% HCl to the compound and watched to see if the compound dissolved. Dissolved 3 drops of the compound in 2 mL of reagent-grade ethanol and added 3 4 5 6 7 8 mL of the 2,4- dinitrophenylhydrazine (2,4-DNP) reagent Added 3 drops of the compound to a test tube then added 5 drops of acetone and 5 drops of chromic acid solution. 9 Added 1.0 ml of 5% ferrous ammonium sulfate solution.to about 4 drops of the compound. Mixed and added 2 drop of 3 M sulfuric acid followed by 20 drops of 2 M KOH in methanol. Mixed thoroughly by shaking and allow to stand for 5 minutes. 22 pts Chemical equation/s of the reaction/s that gave definitive (confirmatory) information about this compound. You must use STRUCTURAL FORMULAS (hand-drawn, soft-ware drawn (NOT copied from the internet! Molecular formulas will not be acceptable and will get no credit. You may disregard the chemical equation for combustion reaction









