Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Upload answer sheets Test time left: 02:03:54 1. The free energy change (AG) accompanying a given process is -85.77 KJ at 25C and -83.68KJ at
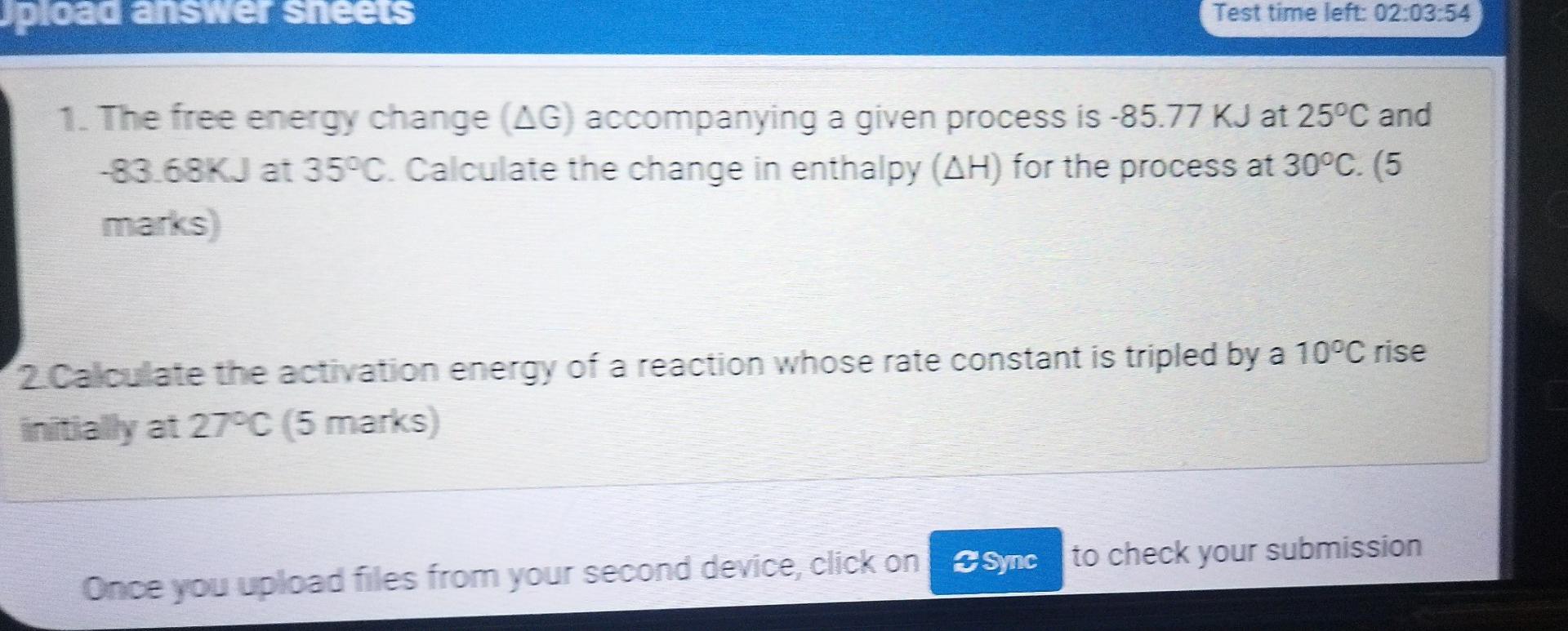
Upload answer sheets Test time left: 02:03:54 1. The free energy change (AG) accompanying a given process is -85.77 KJ at 25C and -83.68KJ at 35C. Calculate the change in enthalpy (AH) for the process at 30C. (5 marks 2 Calculate the activation energy of a reaction whose rate constant is tripled by a 10C rise initially at 27C 5 marks Once you upload files from your second device, click on Sync to check your submission
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


