Answered step by step
Verified Expert Solution
Question
1 Approved Answer
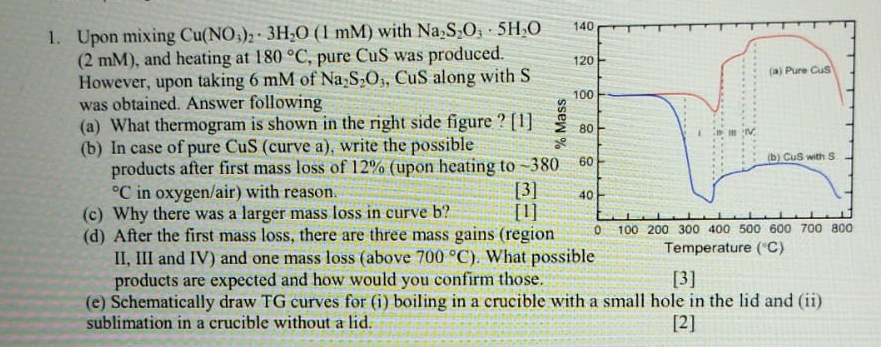
Upon mixing C u ( N O 3 ) 2 * 3 H 2 O ( 1 m M ) with N a 2 S
Upon mixing with and heating at pure CuS was produced. However, upon taking of CuS along with was obtained. Answer following
a What thermogram is shown in the right side figure
b In case of pure CuS curve a write the possible products after first mass loss of upon heating to in oxygenair with reason.
c Why there was a larger mass loss in curve b
d After the first mass loss, there are three mass gains region II III and IV and one mass loss above What possibie products are expected and how would you confirm those.
e Schematically draw TG curves for i boiling in a crucible with a small hole in the lid and ii sublimation in a crucible without a lid.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


