Answered step by step
Verified Expert Solution
Question
1 Approved Answer
URGEBT Chloroform (CHCl3) reacts with hydrochloric acid (HCl) to form methane (ECH4) and chlorine (Cl2). a) Use bond energies to calculate the H for reaction.
URGEBT 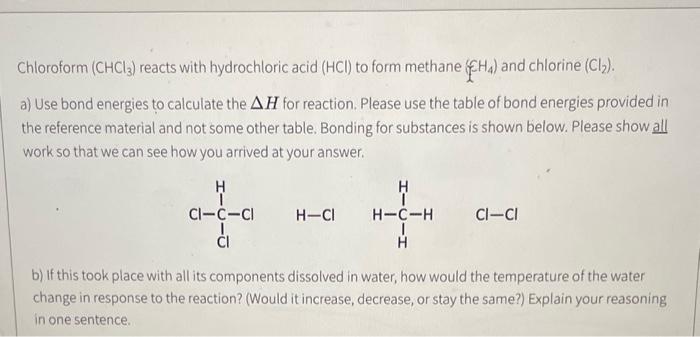
Chloroform (CHCl3) reacts with hydrochloric acid (HCl) to form methane (ECH4) and chlorine (Cl2). a) Use bond energies to calculate the H for reaction. Please use the table of bond energies provided in the reference material and not some other table. Bonding for substances is shown below. Please show all work so that we can see how you arrived at your answer. b) If this took place with all its components dissolved in water, how would the temperature of the water change in response to the reaction? (Would it increase, decrease, or stay the same?) Explain your reasoning in one sentence. Chloroform (CHCl3) reacts with hydrochloric acid (HCl) to form methane (ECH4) and chlorine (Cl2). a) Use bond energies to calculate the H for reaction. Please use the table of bond energies provided in the reference material and not some other table. Bonding for substances is shown below. Please show all work so that we can see how you arrived at your answer. b) If this took place with all its components dissolved in water, how would the temperature of the water change in response to the reaction? (Would it increase, decrease, or stay the same?) Explain your reasoning in one sentence 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


