Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Use chemical equilibrium code Stanjan to calculate and compare the equilibrium adiabatic flame temperatures of the following three fuels: ethanol (C;H;OH), hydrogen (H2) and
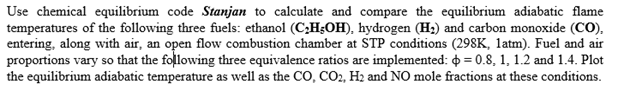
Use chemical equilibrium code Stanjan to calculate and compare the equilibrium adiabatic flame temperatures of the following three fuels: ethanol (C;H;OH), hydrogen (H2) and carbon monoxide (CO), entering, along with air, an open flow combustion chamber at STP conditions (298K, latm). Fuel and air proportions vary so that the following three equivalence ratios are implemented: 0 = 0.8, 1, 1.2 and 1.4. Plot the equilibrium adiabatic temperature as well as the CO, CO2, H2 and NO mole fractions at these conditions.
Step by Step Solution
★★★★★
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
4400 4000 3600 3200 Rich Lean 2800 2400 2000 40 60 100 120 140 160 80 180 200 Combustion air 1 Adiab...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


