Question
Use the diatomic ideal gas heat capacity e-Bhy Cv = R + Nahv G-e-Bhv 1 - e-Bhv Nghv ( a e-Bhy - e-Bhv e-hv/kBT

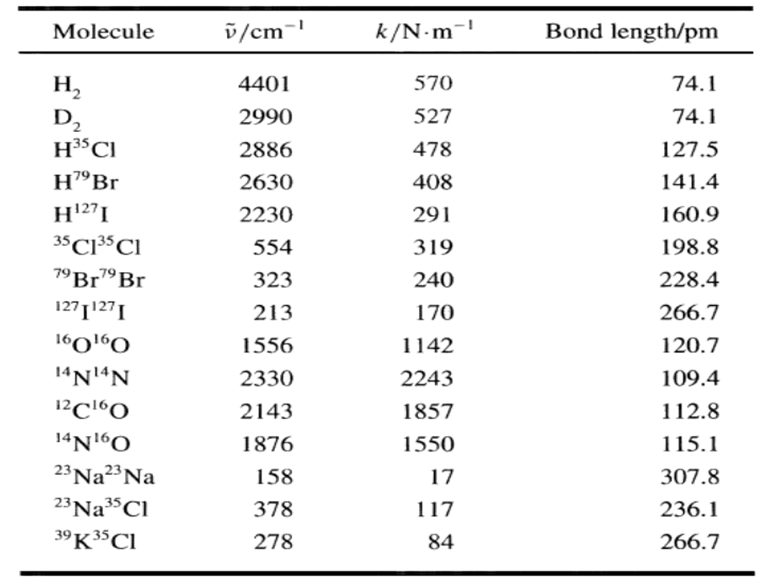
Use the diatomic ideal gas heat capacity e-Bhy Cv = R + Nahv G-e-Bhv 1 - e-Bhv Nghv ( a e-Bhy - e-Bhv e-hv/kBT (1 - e-hv/kBT)2 1 hv kBT + and the value of for HBr given in this table to calculate the equation of the molar heat capacity of HBr in terms of T and R. Cy = II Molecule v/cm-' k/N-m- Bond length/pm 74.1 H, D, 4401 570 2990 527 74.1 H3CI 2886 478 127.5 H"Br 2630 408 141.4 H27I 2230 291 160.9 35 C13CI 554 319 198.8 79 Br79 Br 323 240 228.4 1271127I 213 170 266.7 1601O 1556 1142 120.7 14N14N 2330 2243 109.4 2143 1857 112.8 14NO 1876 1550 115.1 23 Na23 Na 23 Na3Cl 39K35CI 158 17 307.8 378 117 236.1 278 84 266.7
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Answer Solution The wavenumber D for N0 is 1876 cm1 The molar heat capacity Cv is given ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Physics
Authors: Jearl Walker, Halliday Resnick
8th Extended edition
471758019, 978-0471758013
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



