Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Use the Fe-C phase diagram at the end to answer the following questions. A 0.55 wt% carbon steel is slow cooled from 1000C to

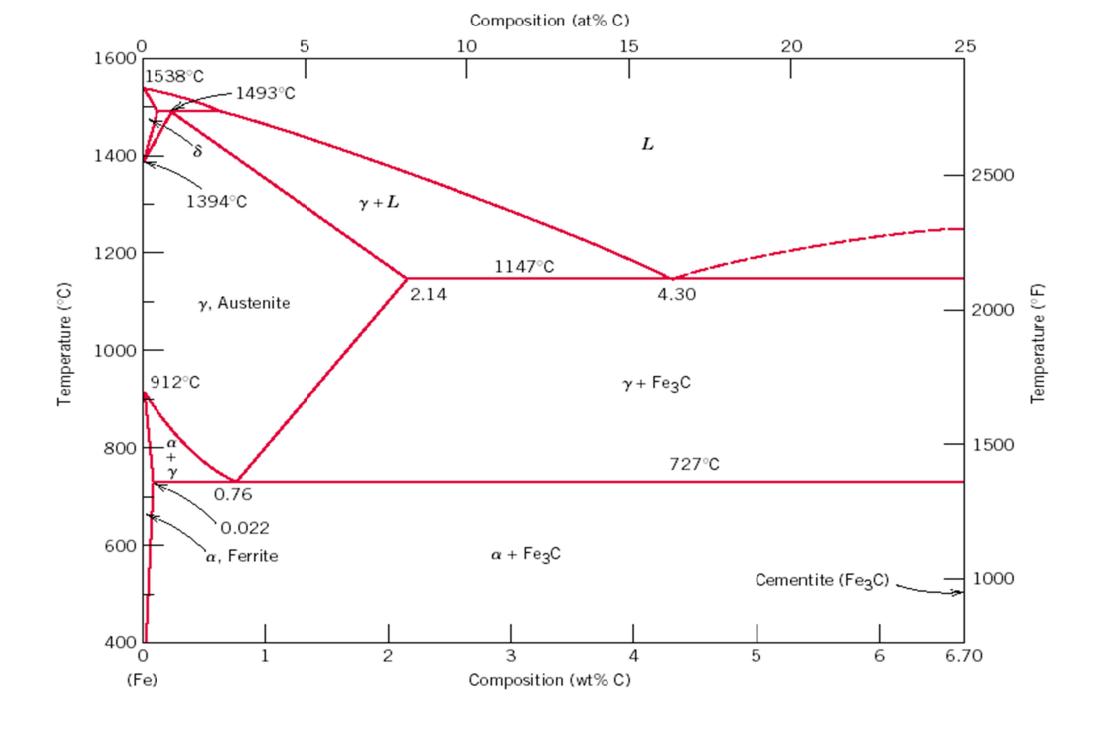

Use the Fe-C phase diagram at the end to answer the following questions. A 0.55 wt% carbon steel is slow cooled from 1000C to a temperature slightly below 727C. In the final structure: (a) Sketch and label a typical microstructure showing all phases & micro-constituents that would be present at 720 C (b) Indicate the phase or phases present and the composition of each phase, and calculate how much of each phase is present. (c) At the same temperature determine the fractions of (proeutectoid) ferrite and pearlite. (d) What phase would be present if the sample is very rapidly quenched instead from 1000C to room temperature? (e) Can this phase be found on the Fe-C diagram, and if not, why not? Temperature (C) 0 1600 1400 1200 1000 1538C 600 800 a 400 0 912C (Fe) +2 8 Y 1493C 1394C y, Austenite 0.76 0.022 a, Ferrite 5 y + L 2 2.14 Composition (at% C) 15 10 1147C a + Fe3C L 3 4 Composition (wt% C) 4.30 y + Fe3C 727C 20 Cementite (Fe3C) 5 6 25 2500 2000 1500 1000 6.70 Temperature (F) 1. Use the Fe-C phase diagram above to answer the following questions. A 0.55 wt% carbon steel is slow cooled from 1000C to a temperature slightly below 727C. In the final structure: a. Sketch and label a typical microstructure showing all phases & micro-constituents that would be present at 720 C. b. Indicate the phase or phases present and the composition of each phase, and calculate how much of each phase is present. c. At the same temperature determine the fractions of (proeutectoid) ferrite and pearlite. d. What phase would be present if the sample is very rapidly quenchedinstead from 1000C to room temperature? e. Can this phase be found on the Fe-C diagram, and if not, why not?
Step by Step Solution
★★★★★
3.49 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


