Answered step by step
Verified Expert Solution
Question
1 Approved Answer
use the ff table for answering Tank 1 containing pure carbon monoxide (A) at 2 atm is connected to another tank, Tank 2, containing pure

use the ff table for answering

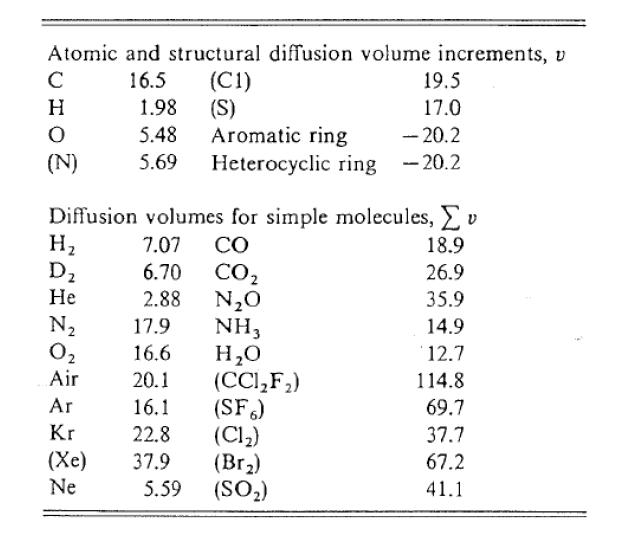

Tank 1 containing pure carbon monoxide (A) at 2 atm is connected to another tank, Tank 2, containing pure hydrogen gas (B) also at 72 kPa. Both tanks are at 15 o C. Initially, the valve located at the 0.72132- in diameter, 200-mm long tube connecting the two tanks is closed to prevent mixing. Both tanks have a volume of 50 cubic feet. However, due to mistakes by the ones monitoring the tanks, the valve was inadvertently opened and mixing of the gases commences. At the time they noticed that the valve was open, the mass fraction of CO in one tank is 0.49987 and the valve was immediately closed. Assume ideal gas behavior. (Atomic weights: C-12.00, H-1.00, 0-16.00) Dco-H Determine using the appropriate correlation. What is the mole fraction of H2 in Tank 1 after the valve was closed, assuming the tube volume is negligible? What is the total molar rate of diffusion of H2 through the tube right before the valve was closed in mol/s? Towards which tank is H2 diffusing?
Step by Step Solution
★★★★★
3.37 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Solution Tank 1 Carb...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


