Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Questions and Problems Q1 How many ml. of a 0.10 M NaOH solution are needed on neutralize 15 ml of u 9.20 M H.PO,
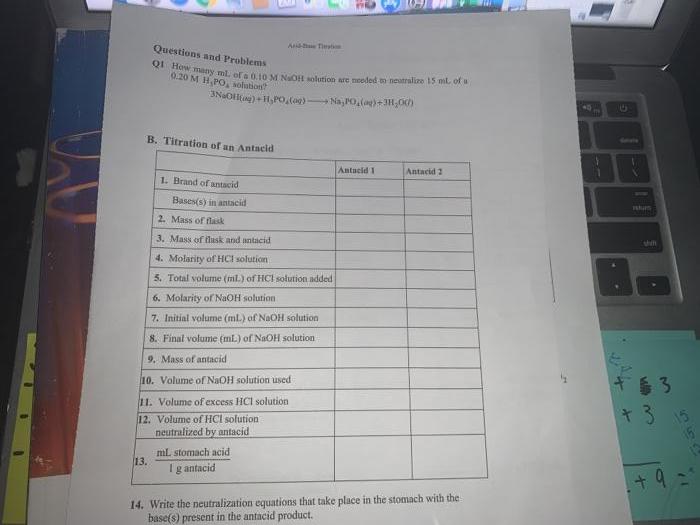
Questions and Problems Q1 How many ml. of a 0.10 M NaOH solution are needed on neutralize 15 ml of u 9.20 M H.PO, solution B. Titration of an Antacid 13. 3NaOH(aq) +H,PO (og)Na,PO(g) + 3H00) 1. Brand of antacid Bases(s) in antacid Ad-Te 2. Mass of flask 3. Mass of flask and antacid 4. Molarity of HCI solution 5. Total volume (ml.) of HCl solution added 6. Molarity of NaOH solution 7. Initial volume (ml.) of NaOH solution 8. Final volume (mL) of NaOH solution 9. Mass of antacid 10. Volume of NaOH solution used 11. Volume of excess HCl solution 12. Volume of HCI solution neutralized by antacid ml stomach acid Ig antacid Antacid 1 Antacid 2 14. Write the neutralization equations that take place in the stomach with the base(s) present in the antacid product. 1 1 shim 63 +3 552 1 + 9 =
Step by Step Solution
★★★★★
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
SO Q1 Given NaOH 010M 1dlg NaOH HPO4 02017 Vol of Hp04 15mL Naz P...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


