Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Use the standard electrode potentials given in the following table to answer the questions below: Half-Reaction E V Lit + Li |-3,04 e- Mg2+
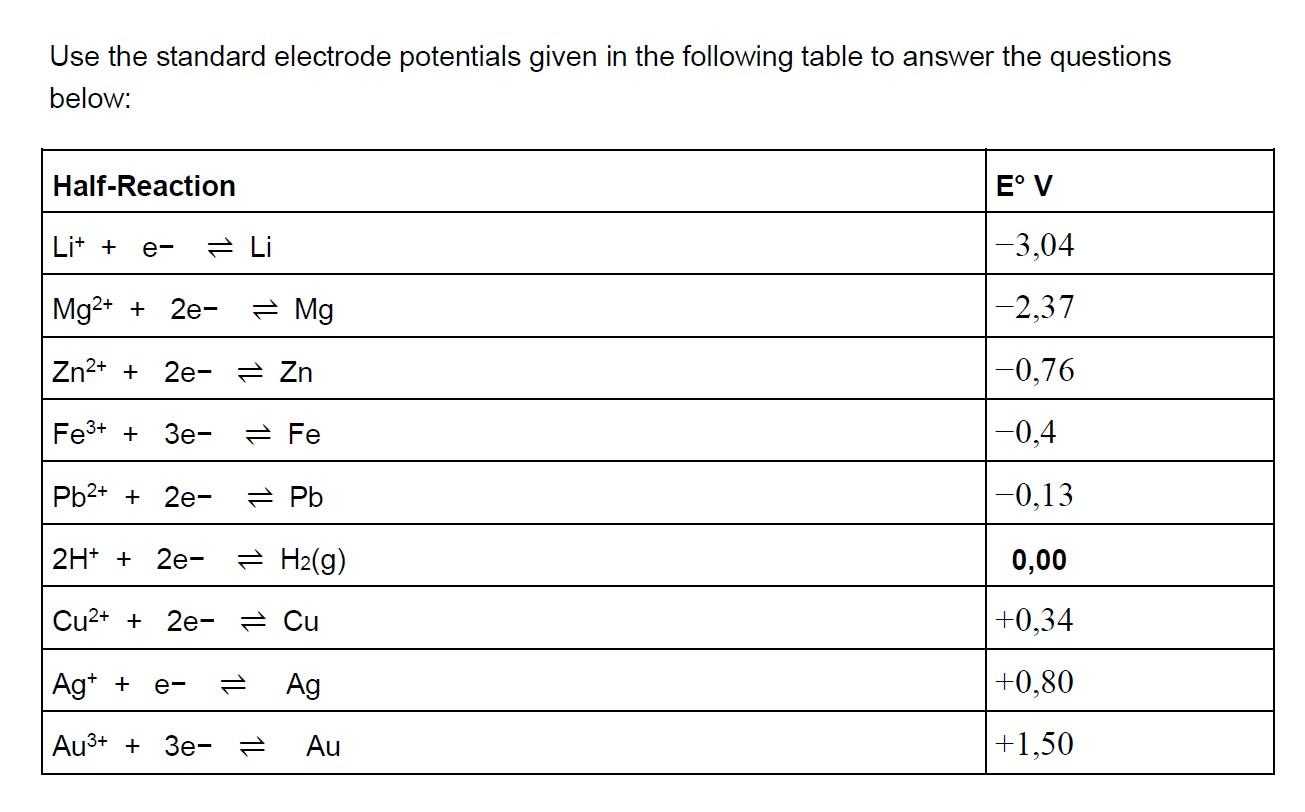

Use the standard electrode potentials given in the following table to answer the questions below: Half-Reaction E V Lit + Li |-3,04 e- Mg2+ + 2e- Mg -2,37 Zn2+ + 2e- = Zn |-0,76 Fe3+ + 3e- 2 Fe -0,4 Pb2+ + 2e- Pb -0,13 2H* + 2e- = H2(g) 0,00 Cu2+ + 2e- 2 Cu +0,34 Ag* + Ag +0,80 e- Au3+ + 3e- = Au +1,50 (a) Which of the metals on the list can reduce Mg2*(aq) to Mg(s)? Explain your answer. (3) (b) Which of the metals on the list is most readily reduced? Explain your answer. (3) (c) Provide a balanced chemical equation using the half-reaction method for the reaction of Ag*(aq) with Zn(s). Show all the steps and determine if the reaction be spontaneous (product-favoured). (8) (d) Predict the chemical reactions that will occur at the two electrodes in the electrolysis of an aqueous sodium hydroxide solution. What is the minimum voltage needed for this reaction to take place? (8)
Step by Step Solution
★★★★★
3.39 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635fb7ff36fac_233062.pdf
180 KBs PDF File
635fb7ff36fac_233062.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


