Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Useful Equations - qsys=0= qrxn + qheating q=C (Tf - Ti) = CAT Csolution ( C-1) = MH2O (g) x SH20 (1g-1 C-1) qheating
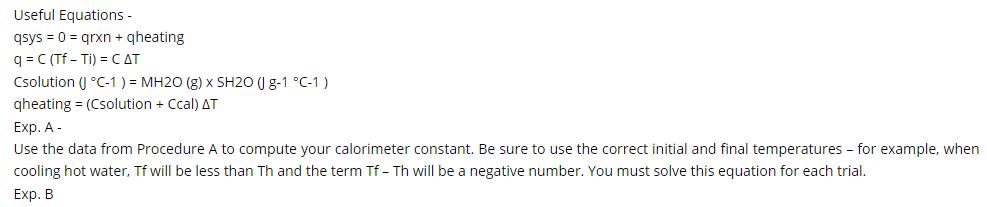
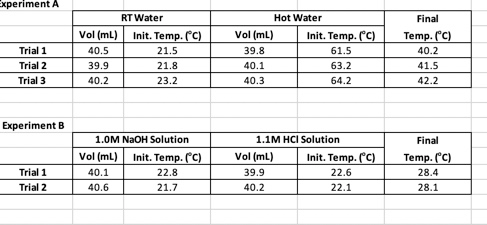

Useful Equations - qsys=0= qrxn + qheating q=C (Tf - Ti) = CAT Csolution ( C-1) = MH2O (g) x SH20 (1g-1 C-1) qheating = (Csolution + Ccal) AT Exp. A- Use the data from Procedure A to compute your calorimeter constant. Be sure to use the correct initial and final temperatures - for example, when cooling hot water, Tf will be less than Th and the term Tf - Th will be a negative number. You must solve this equation for each trial. Exp. B Experiment A Trial 1 Trial 2 Trial 3 Experiment B Trial 1 Trial 2 Vol (ml) 40.5 39.9 40.2 RT Water 40.1 40.6 Init. Temp. (C) 21.5 21.8 23.2 1.0M NaOH Solution Vol (ml) Init. Temp. (C) 22.8 21.7 Vol (ml) 39.8 40.1 40.3 Vol (ml) Hot Water 1.1M HCI Solution 39.9 40.2 Init. Temp. (C) 61.5 63.2 64.2 Init. Temp. (C) 22.6 22.1 Final Temp. (C) 40.2 41.5 42.2 Final Temp. (C) 28.4 28.1 Exp. B- qsys = 0 = qrxn + qwater+qcalorimeter (7) Use equation 7 to define each q in the system with measured (or provided) parameters using the same variables as previously (MH2O, Tf, Ti, and Ccal). Use the data from your first trial and the Ccal value found in Experiment A to determine your experimental qrxn. Calculate AHrxn (in kJ/mol) based on the available concentration information and the number of mols of reaction that occur to give off qrxn. Assume the specific heat and density of the solution are the same as for pure water. Be sure to use your actual volumes and concentrations. Repeat the calculation for your second trial and average these two values to report the AHrxn.
Step by Step Solution
★★★★★
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
We can use the following formula Q mcT Q heat energy Joules J m mass of a substance g c specific heat units JgC is a symbol meaning the change in T ch...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


