Question
Useful I data: Y = C/ C 1.4 Gas Molar mass- M-, kg/k mol Air 28.97 32 1.4 en Butane (C,H10) Nitrogen (N2) Methane
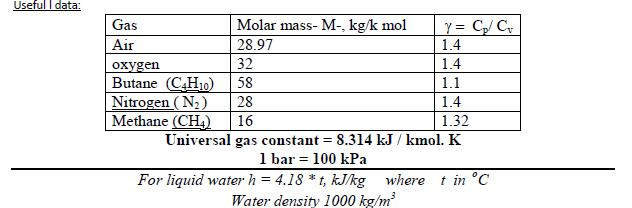

Useful I data: Y = C/ C 1.4 Gas Molar mass- M-, kg/k mol Air 28.97 32 1.4 en Butane (C,H10) Nitrogen (N2) Methane (CH) 58 1.1 28 1.4 16 1.32 Universal gas constant = 8.314 kJ / kmol. K 1 bar = 100 kPa For liquid water h = 4.18 *t, kJ/kg where t in C Water density 1000 kg/m 10- Methane (CH,) gas enters a horizontal, well-insulated nozzle operating at steady state at 80C and a velocity of 10 m/s. Assuming ideal gas behavior for the methane, plot the temperature of the gas exiting the nozzle, in C, versus the exit velocity ranging from 500 to 600 m/s.
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



