Question: A reversible power cycle receives energy Q h from a reservoir at temperature T h and rejects Q c to a reservoir at temperature T
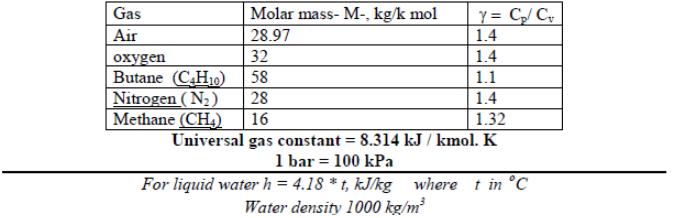
y = C/C 1.4 Gas Molar mass- M-, kg/k mol Air 28.97 1.4 32 Butane (CH1o) en 58 1.1 Nitrogen (N2) 28 1.4 Methane (CH,) 16 1.32 Universal gas constant = 8.314 kJ / kmol. K 1 bar = 100 kPa %3! where t in C For liquid water h = 4.18 *t, kJ/kg Water density 1000 kg/m
Step by Step Solution
3.53 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


