Question: Using Chapter 1: Faraday titled On the Absolute Quantity of Electricity Associated With the Particles or Atoms of Matter by Michael Faraday. -What is the
Using Chapter 1: Faraday titled "On the Absolute Quantity of Electricity Associated With the Particles or Atoms of Matter" by Michael Faraday.
-What is the main idea of the Author's argument? What is the main essence of the peice?
-What ways does the author use to propose and support their argument? What is the underlying structure and evidence used?
-What are the strengths and limitations of the authors argument?
-How can the findings and documented work be simplified?





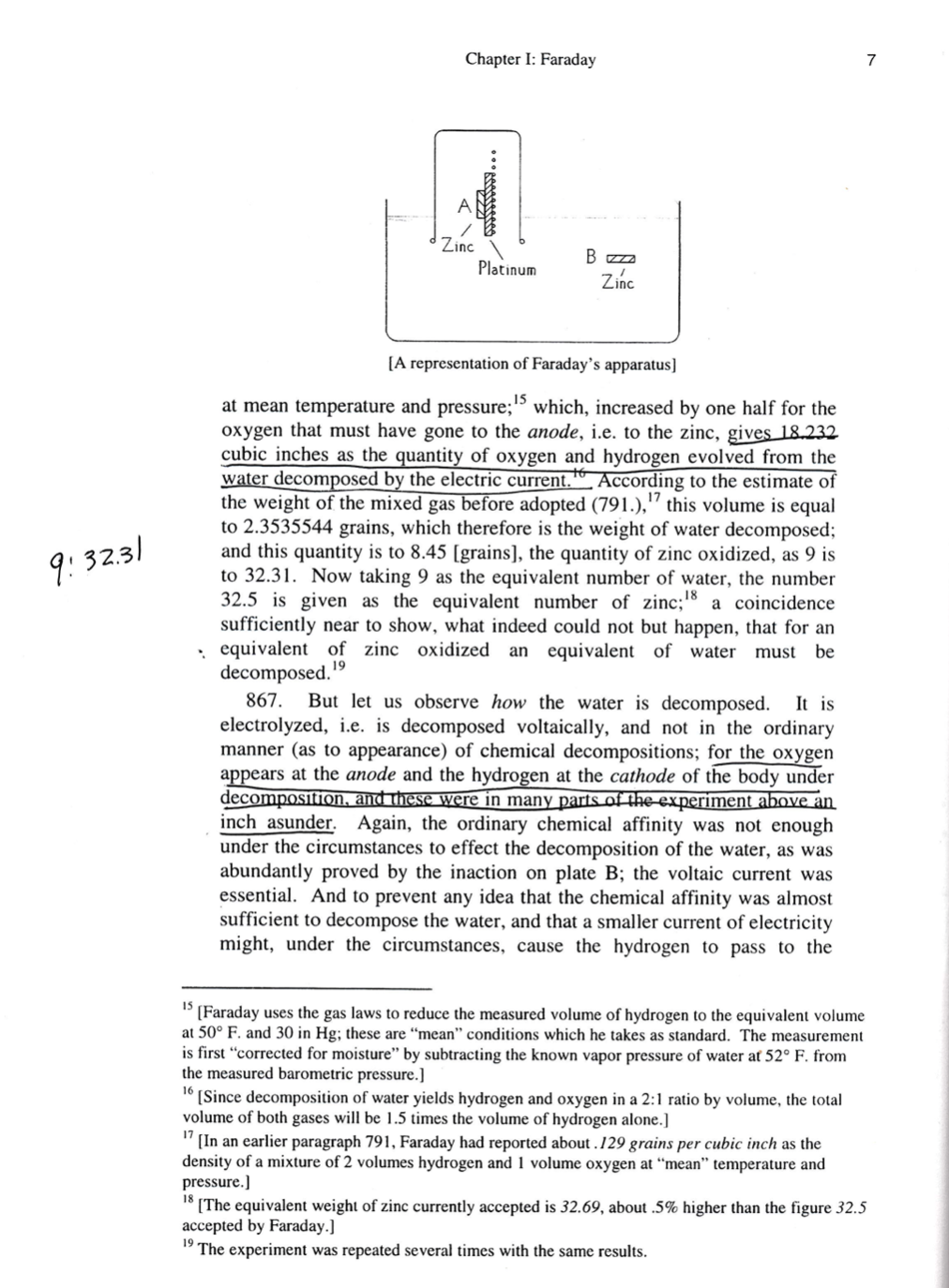



2 Chapter I: Faraday On The Absolute Quantity Of Electricity Associated With The Particles Or Atoms Of Matter.' Michael Faraday 852. The theory of definite electrolytical or electro-chemical action appears to me to touch immediately upon the absolute quantity of electricity or electric power belonging to different bodies. It is impossible, perhaps, to speak on this point without committing oneself beyond what present facts will sustain; and yet it is equally impossible, and perhaps would be impolitic, not to reason upon the subject. Although we know nothing of what an atom is, yet we cannot resist forming some idea of a small particle, which represents it to the mind; and though we are in equal, if not greater, ignorance of electricity, so as to be unable to say whether it is a particular matter or matters, or mere motion of ordinary matter, or some third kind of power or agent, yet there is an immensity of facts which justify us in believing that the atoms of matter are in some way endowed or associated with electrical powers, to which they owe their most striking qualities, and amongst them their mutual chemical affinity. As soon as we perceive, through the teaching of Dalton, that chemical powers are, however varied the circumstances in which they are exerted, definite for each body, we learn to estimate the relative degree of force which resides in such bodies: and when upon that knowledge comes the fact, that the electricity, which we appear to be capable of loosening from its habitation for awhile, and conveying from place to place, whilst it retains its chemical force, can be measured out, and being so measured out is found to be as definite in its action as any of those portions which, remaining associated with the particles of matter, give them their chemical relation; we seem to have found the link which connects the proportion of that we have evolved to the proportion of that belonging to the particles in their natural state. 853. Now it is wonderful to observe how small a quantity of a compound body is decomposed by a certain portion of electricity. Let us, for instance, consider this and a few other points in relation to water. One grain of water, acidulated to facilitate conduction, will require an electric current to be continued for three minutes and three quarters of t = 3-2 Minutes time to effect its decomposition, which current must be powerful enough my= to retain a platina wire 1/104 of an inch in thickness,' red hot, in the air [Experimental Researches in Electricity, VIIth series, section 13. Dover edition, Vol.I, pp. 249ff.] [One grain is very nearly .0648 grams.]Chapter I: Faraday 3 during the whole time; and if interrupted anywhere by charcoal points, will produce a very brilliant and constant star of light. If attention be paid to the instantaneous discharge of electricity of tension, as illustrated in the beautiful experiments of Mr. Wheatstone," and to what I have said elsewhere on the relation of common and voltaic electricity (371. 375.), it will not be too much to say that this necessary quantity of electricity is equal to a very powerful flash of lightning. Yet we have it under perfect command; can evolve, direct, and employ it at pleasure; and when it has performed its full work of electrolyzetion, it has only separated the elements of a single grain of water. 854. On the other hand, the relation between the conduction of the electricity and the decomposition of the water is so close, that one cannot take place without the other. If the water is altered only in that small degree which consists in its having the solid instead of the fluid state. the a thermal connery conduction is stopped, and the decomposition is stopped with it. with it Whether the conduction be considered as depending upon the decomposition, or not (413. 703.), still the relation of the two functions is equally intimate and inseparable. 855. Considering this close and twofold relation, namely, that without decomposition transmission of electricity does not occur; and, that for a given definite quantity of electricity passed, an equally definite and constant quantity of water or other matter is decomposed; considering also that the agent, which is electricity, is simply employed in overcoming electrical powers in the body subjected to its action: it seems a probable, and almost a natural consequence, that the quantity which passes is the equivalent of, and therefore equal to, that of the particles separated; 1.e. that if the electrical power which holds the elements of a grain of water in combination, or which makes a grain of oxygen and hydrogen in the right proportions unite into water when they are made to combine, could be thrown into the condition of a current, it would exactly equal the current required for the separation of that grain of water into its elements again. 856. This view of the subject gives an almost overwhelming idea of the extraordinary quantity or degree of electric power which naturally theory, that it is indifferent. The same quantity of electricity which, passed in a given time, can heat an inch of platina wire of a certain diameter red hot, can also heat a hundred, a thousand, or any length of the same wire to the same degree, provided the cooling circumstances are the same for every part in all cases. This I have proved by the volta-electrometer. I found that whether half an inch or eight inches were retained at one constant temperature of dull redness, equal quantities of water were decomposed in equal times. When the half-inch was used, only the center portion of wire was ignited. A fine wire may even be used as a rough but ready regulator of a voltaic current; for if it be made part of the circuit, and the larger wires communicating with it be shifted nearer to or further apart, so as to keep the portion of wire in the circuit sensibly at the same temperature, the current passing through it will be nearly uniform. Literary Gazette, 1833, March I and 8. Philosophical magazine, 1833, p. 204. L'Institute, 1833, p. 261 [Faraday refers to numbered paragraphs in earlier series of the Experimental Researches.]Chapter I: Faraday belongs to the particles of matter; but it is not inconsistent in the slightest degree with the facts which can be brought to bear on this point. To illustrate this I must say a few words on the voltaic pile." 857. Intending hereafter to apply the results given in this and the preceding series of Researches to a close investigation of the source of electricity in the voltaic instrument, I have refrained from forming any decided opinion on the subject; and without at all meaning to dismiss metallic contact, or the contact of dissimilar substances, being conductors, but not metallic, as if they had nothing to do with the origin of the current, I still am fully of the opinion with Davy, that it is at least continued by chemical action, and that the supply constituting the current is almost entirely from that source. 858. Those bodies which, being interposed between the metals of the voltaic pile, render it active, are all of them electrolytes (476.); and it cannot but press upon the attention of every one engaged in considering this subject, that in those bodies (so essential to the pile) decomposition and the transmission of a current are so intimately connected, that one cannot happen without the other. This I have shown abundantly in water, and in numerous other cases (402. 476.). If, then, a voltaic trough have its extremities connected by a body capable of being decomposed, as water, we shall have a continuous current through the apparatus; and whilst it remains in this state we may look at the part where the acid is acting upon the plates, and that where current is acting upon the water, as the reciprocals of each other. In both parts we have the two conditions inseparable in such bodies as these, namely, the passing of a current, and decomposition; and this is as true of the cells in the battery as of the water cell; for no voltaic battery has as yet been constructed in which the chemical action is only that of combination: decomposition is always included, and is, I believe, an essential chemical part. 859. But the difference in the two parts of the connected battery, that is, the decomposition or experimental cell, and the acting cells, is simply this. In the former we urge the current through, but it, apparently of necessity, is accompanied by decomposition: in the latter we cause decompositions by ordinary chemical actions (which are, however, themselves electrical), and, as a consequence, have the electrical current; and as the decomposition dependent upon the current is definite in the former case, so is the current associated with the decomposition also definite in the latter (862. &c.). 860. Let us apply this in support of what I have surmised respecting the enormous electric power of each particle or atom of matter (856.). I " By the term voltaic pile, I mean such apparatus or arrangement of metals as up to this time have been called so, and which contain water, brine, acids, or other aqueous solutions or decomposable substances (476.), between their plates. Other kinds of electric apparatus may hereafter be invented, and I hope to construct some not belonging to the class of instruments discovered by Volta. [Note: The voltaic pile is an instance of what we know as the electric battery; and indeed Faraday also uses the term "battery" in paragraphs 858ff. below.]Chapter I: Faraday 5 showed in a former series of these Researches on the relation by measure of common and voltaic electricity,' that two wires, one of platina and one of zinc, each one eighteenth of an inch in diameter, placed five- sixteenths of an inch apart, and immersed to the depth of five eighths of an inch in acid, consisting of one drop of oil of vitriol and four ounces of distilled water at a temperature of about 60 Fahr., and connected at the other extremities by a copper wire eighteen feet long, and one eighteenth ITG C. of an inch in thickness, yielded as much electricity in little more than three seconds of time as a Leyden battery charged by thirty turns of a very large and powerful plate electric machine in full action (371.). This quantity, though sufficient if passed through the head of a rat or cat to have killed it, as by a flash of lightning, was evolved by the mutual action of so small a portion of the zinc wire and water in contact with it, that the loss of weight sustained by either would be inappreciable by our most delicate instruments; and as to the water which could be decomposed by that current, it must have been insensible in quantity, for no trace of hydrogen appeared upon the surface of the platina during those three seconds. 861. What an enormous quantity of electricity, therefore, is required for the decomposition of a single grain of water! We have already seen that it must be in quantity sufficient to sustain a platina wire 1/104 of an inch in thickness, red hot, in contact with the air, for three minutes and three quarters (853.), a quantity which is almost infinitely greater than that which could be evolved by the little standard voltaic arrangement to which I have just referred (860. 371.). I have endeavored to make a comparison by the loss of weight of such a wire in a given time in such an acid, according to a principle and experiment to be almost immediately described (862.); but the proportion is so high that I am almost afraid to mention it. It would appear that 800,000 such charges of Mechanical + the Leyden battery as I have referred to above, would be necessary to Electrical - supply electricity sufficient to decompose a single grain of water; or, if I am right, to equal the quantity of electricity which is naturally associated Chemical with the elements of that grain of water, endowing them with their mutual chemical affinity. 862. In further proof of this high electric condition of the particles of matter, and the identity as to quantity of that belonging to them with that necessary for their separation, I will describe an experiment of great simplicity but extreme beauty, when viewed in relation to the evolution of an electric current and its decomposing powers. [By "common" electricity Faraday means static electricity; while "voltaic" electricity is what is produced by the voltaic battery. Until Faraday showed their equivalence, it was uncertain whether the two "electricities" were the same or different.] [The eighteen-foot length of wire formed the coil of a galvanometer. See Experimental Researches, Vol. I, p. 105.] ["Leyden battery": an array (a "battery") of Leyden jars.]Chapter 1: Faraday 863. A dilute sulphuric acid, made by adding about one part by measure of oil of vitriol to thirty parts of water, will act energetically upon a piece of zinc plate in its ordinary and simple state: but, as Mr. Sturgeon has shewn,\" not at all. or scarcely so. if the surface 0 the metal has in the rst instance e a ted zinc will act powerfully with platina as an electrornotor.ll hydrogen being evolved on the sur ace 0 e atter meta . as t e ' ' oxidized and dissolved. The amalgamation is best effected by sprinkling a few drops of mercury upon the surface of the zinc. the latter being moistened with the dilute acid. and rubbing with the ngers or tow so as to extend the liquid metal over the whole of the surface. Any mercury in excess. forming liquid drops upon the zinc, should be wiped off.\" 864. Two plates of zinc thus amalgamated were dried and accurately weighed; one. which we shall call A, weighed 163.1 grains; the other. to be called B, weighed 148.3 grains.\" They were about ve inches long, and 0.4 of an inch wide. An earthenware pneumatic trough was lled with dilute sulphuric acid. of the strength just described (863.), and a gas jar. also lled with the acid. inverted in it.\" A plate of platina of nearly the same length, but about three times as wide as the zinc plates. was put up into this jar. The zinc plate A was also introduced into the jar, and brought in contact with the platina. and at the same moment the plate B was put into the acid of the trough, but out of contact with other metallic matter. 865. Strong action immediately occurred in the jar upon the contact of the zinc and platina plates. Hydrogen gas rose from the platina, and was collected in the jar. but no hydrogen or other gas rose from either zinc plate. In about ten or twelve minutes. sufcient hydrogen having been collected, the experiment was stopped; during its progress a__fe_\\_v_ W The '3 . p.195\". plates were was e m tstt ed water. dried. and reweighed. Plate B weighed 148.3 grains. as before. having lost nothing by the direct chemical action of the acid. Plate A weighed 154.65 grains. 8.45 grains of it having been oxidized and dissolved during the experiment. 866. The hydrogen gas was next transferred to a water-trough and measured; it amounted to 12.5 cubic inches, the temperature being 52. and the barometer 29.2 inches. This quantity, corrected for temperature. pressure. and moisture, becomes 12.15453 cubic inches of dry hydrogen "3 Recent Experimental Researches. &c.. 1830. p. 74. &c. \" ["electromotor": something that moves or tends to move electricity (1827).} '2 The experiment may be made with pure zinc. which. as chemists well know. is but slightly acted upon by dilute sulphuric acid in comparison with ordinary zinc. which during the actiOn is subject to an innity of voltaic actions. Sec Dela Rive on this subject. Bibliotheque Universelle. 1830. p. 391. 1" [0r. since one grain very nearly equals .0648 grams. plate A weighs 10.5639 grams and plate B weighs 9.6098 grams.] \" The acid was left during a night with a small piece of unamalgamated zinc in it. for the purpose of evolving such air as might be inclined to separate. and bringing the whole into a constant state. Chapter I: Faraday 7 A Zinc Platinum Zinc [A representation of Faraday's apparatus] at mean temperature and pressure;" which, increased by one half for the oxygen that must have gone to the anode, i.e. to the zinc, gives 18.232 cubic inches as the quantity of oxygen and hydrogen evolved from the water decomposed by the electric current. According to the estimate of the weight of the mixed gas before adopted (791.), "this volume is equal to 2.3535544 grains, which therefore is the weight of water decomposed; 9: 32.31 and this quantity is to 8.45 [grains], the quantity of zinc oxidized, as 9 is to 32.31. Now taking 9 as the equivalent number of water, the number 32.5 is given as the equivalent number of zinc;" c: " a coincidence sufficiently near to show, what indeed could not but happen, that for an equivalent of zinc oxidized an equivalent of water must be decomposed. 19 867. But let us observe how the water is decomposed. It is electrolyzed, i.e. is decomposed voltaically, and not in the ordinary manner (as to appearance) of chemical decompositions; for the oxygen appears at the anode and the hydrogen at the cathode of the body under decomposition, and these were in many parts of the experiment above an inch asunder. Again, the ordinary chemical affinity was not enough under the circumstances to effect the decomposition of the water, as was abundantly proved by the inaction on plate B; the voltaic current was essential. And to prevent any idea that the chemical affinity was almost sufficient to decompose the water, and that a smaller current of electricity might, under the circumstances, cause the hydrogen to pass to the [Faraday uses the gas laws to reduce the measured volume of hydrogen to the equivalent volume at 50 F. and 30 in Hg; these are "mean" conditions which he takes as standard. The measurement is first "corrected for moisture" by subtracting the known vapor pressure of water at 52 F. from the measured barometric pressure.] [Since decomposition of water yields hydrogen and oxygen in a 2:1 ratio by volume, the total volume of both gases will be 1.5 times the volume of hydrogen alone.] "[In an earlier paragraph 791, Faraday had reported about . 129 grains per cubic inch as the density of a mixture of 2 volumes hydrogen and 1 volume oxygen at "mean" temperature and pressure.] [The equivalent weight of zinc currently accepted is 32.69, about .5% higher than the figure 32.5 accepted by Faraday.] The experiment was repeated several times with the same results.Co Chapter I: Faraday cathode, I need only refer to the results which I have given (807, 813), to shew that the chemical action at the electrodes has not the slightest influence over the quantities of water or other substances decomposed between them, but that they are entirely dependent upon the quantity of electricity which passes. 868. What, then, follows as a necessary consequence of the whole experiment? Why, this: that the chemical action upon 32.31 parts, or one equivalent of zinc, in this simple voltaic circle, was able to evolve such quantity of electricity in the form of a current as, passing through water, should decompose 9 parts, or one equivalent of that substance: and considering the definite relations of electricity as developed in the preceding parts of the present paper, the results prove that the quantity of electricity which, being naturally associated with the particles of matter, gives them their combining power, is able, when thrown into a current, to separate those particles from their state of combination; or, in other words, that the electricity which decomposes, and that which is evolved by the decomposition of a certain quantity of matter, are alike. 869. The harmony which this theory of the definite evolution and the equivalent definite action of electricity introduces into the associated theories of definite proportions and electro-chemical affinity, is very great. According to it, the equivalent weights of bodies are simply those quantities of them which contain equal quantities of electricity, or have naturally equal electric powers; it being the ELECTRICITY which determines the equivalent number, because it determines the combining force. Or, if we adopt the atomic theory or phraseology, then the atoms of bodies which are equivalent to each other in their ordinary chemical action, have equal quantities of electricity naturally associated with them. But I must confess I am jealous of the term atom; for though it is very TA easy to talk of atoms, it is very difficult to form a clear idea of their nature, especially when compound bodies are under consideration. 870. I cannot refrain from recalling here the beautiful idea put forth, I believe, by Berzelius (703.) in his development of his views of the electro-chemical theory of affinity, that the heat and light evolved during photoelectric cases of powerful combination are the consequence of the electric effect discharge which is at the moment taking place. The idea is in perfect accordance with the view I have taken of the quantity of electricity associated with the particles of matter. 871. In this exposition of the law of the definite action of electricity, and its corresponding definite proportion in the particles of bodies, I do not pretend to have brought, as yet, every case of chemical or electro- chemical action under its dominion. There are numerous considerations of a theoretical nature, especially respecting the compound particles of matter and the resulting electrical forces which they ought to possess, which I hope will gradually receive their development; and there are 20 ["jealous:" here, suspicious.]Chapter I: Faraday 9 numerous experimental cases, as, for instance, those of compounds formed by weak affinities, the simultaneous decomposition of water and salts, &c., which still require investigation. But whatever the results on these and numerous other points may be, I do not believe that the facts which I have advanced, or even the general laws deduced from them, will suffer any serious change; and they are of sufficient importance to justify their publication, though much may yet remain imperfect or undone. Indeed, it is the great beauty of our science, CHEMISTRY, that advancement in it, whether in a degree great or small, instead of exhausting the subjects of research, opens the doors to further and more abundant knowledge, overflowing with beauty and utility, to those who will be at the easy personal pains of undertaking its experimental investigation. 872. The definite production of electricity (868.) in association with its definite action proves, I think, that the current of electricity in the voltaic pile is sustained by chemical decomposition, or rather by chemical action, and not by contact only. But here, as elsewhere (857.), I beg to reserve my opinion as to the real action of contact, not having yet been able to make up my mind as to whether it is an exciting cause of the current, or merely necessary to allow of the conduction of electricity, otherwise generated, from one metal to the other. 873. But admitting that chemical action is the source of electricity, what an infinitely small fraction of that which is active do we employ in our voltaic batteries! Zinc and platina wires, one eighteenth of an inch in diameter and about half an inch long, dipped into dilute sulphuric acid, so weak that it is not sensibly sour to the tongue, or scarcely to our most delicate test papers, will evolve more electricity in one twentieth of a minute (860.) than any man would willingly allow to pass through his body at once. The chemical action of a grain of water upon four grains of zinc can evolve electricity equal in quantity to that of a powerful thunder-storm (868. 861.). Nor is it merely true that the quantity is active; it can be directed and made to perform its full equivalent duty (867. &c.). Is there not, then, great reason to hope and believe that, by a closer experimental investigation of the principles which govern the development and action of this subtile agent, we shall be able to increase the power of our batteries, or invent new instruments which shall a thousandfold surpass in energy those which we at present possess? 874. Here for a while I must leave the consideration of the definite chemical action of electricity. But before I dismiss this series of experimental Researches, I would call to mind that, in a former series, I showed the current of electricity was also definite in its magnetic action (216. 366. 367. 376. 377.); and, though this result was not pursued to any extent, I have no doubt that the success which has attended the development of the chemical effects is not more than would accompany an investigation of the magnetic phenomena. Royal Institution, December 3Ist, 1833.\\ 10 Chapter I: Faraday A Note on Chemical Equivalence. Faraday confesses he is \"jealous," that is. suspicious, of the term atom. When he wrote in 1833, there was no agreement among natural philosophers as to a tormula for, say, water. which would specify the atomic constituents of a single smallest particle (molecule) of water: was it H0, or H20, or something else? What Faraday was sure of was that chemical substances reacted in definite proportions by weight and, further, that the weights of various substances reacting with one another formed a series of \"equivalent\" or "combining" weights. And since oxygen combines with so many different substances, taking oxygen as the standard of comparison allowed indirect extension of the series to include all elements. For example. 8 parts by weight of oxygen will combine with 1 part by weight of hydrogen (to forth water). But 8 parts by weight of oxygen will also combine with 32.69 parts by weight of zinc (to form zinc oxide). Then the 32.69 of zinc, the 8 of oxygen, and the 1 of hydrogen are all said to be "equivalent" weightsequivalent. that is to say. in combining powerbecause either any two of those quantities will combine with one another or each will combine with the specified weight of the remaining substance. Equivalent weights are relative only to one another and may therefore be expressed in arbitrary units of Weight. But it is particularly convenient to express them in grams, with 8 grams of oxygen taken as the standard. The figures so obtained are called, not merely equivalent weights, but gram-equivalent weights; thus 1 gram. 6 grams, and 32.69 grams are the gram-equivalent weights of hydrogen, oxygen. and zinc, respectively. If. like Faraday, one is skeptical of the atomic view, there cannot be assumed any natural "unit" of chemical combining power. Thus it is for him illuminating in the highest degree to be able to interpret the chemically equivalent weights of various substances as amounts which contain "equal quantities of electricity" (cf. his paragraph 869 above). On the other hand. it the atomic view is accepted, there are consequences even more far-reaching. The molecular formulas finally propounded by Cannizzaro (A Course in Chemical Philosophy, 1859) show that one atom of an element may hold in combination one, two or more atoms of other elements by establishing an integral number of atomic bonds, where the bond to a hydrogen atom is taken as unit.21 2' The number of bonds an atom has formed is known as its valence. Thus in H20 (water) and NH] (ammonia). the oxygen atom is said to be bivalent and the nitrogen atom tervalent. Some elements combine under different conditions to form more than one compound, allowing a single element to exhibit multiple valences. For example. a carbon atom is bivalent in CO but quadrivalent in C02 . Since \"equivalent weights\" of elements are amounts which display the same combining power, then on the atomic view they must also be amounts that form the same total number of atomic bonds. But any number of bivalent atoms will form as many bonds as twice that number of univalent atoms; and in general, equivalent weights
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


