Using excel please plot for Question 2, then please do 3,4,5,6,7
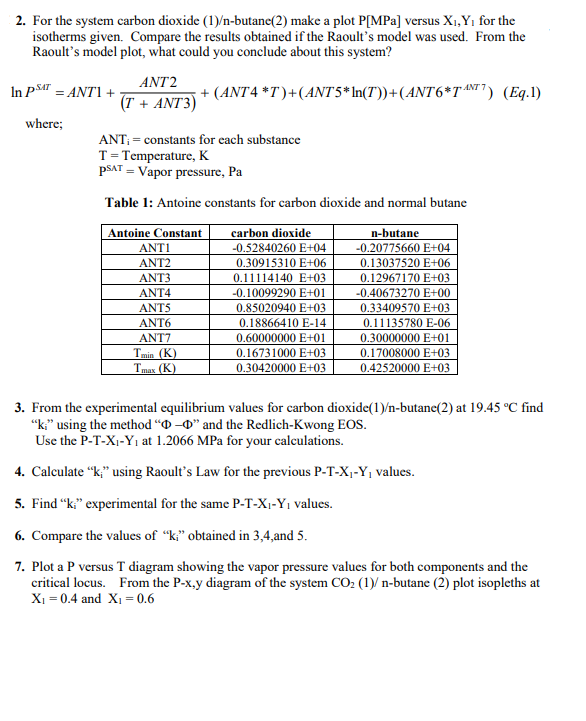
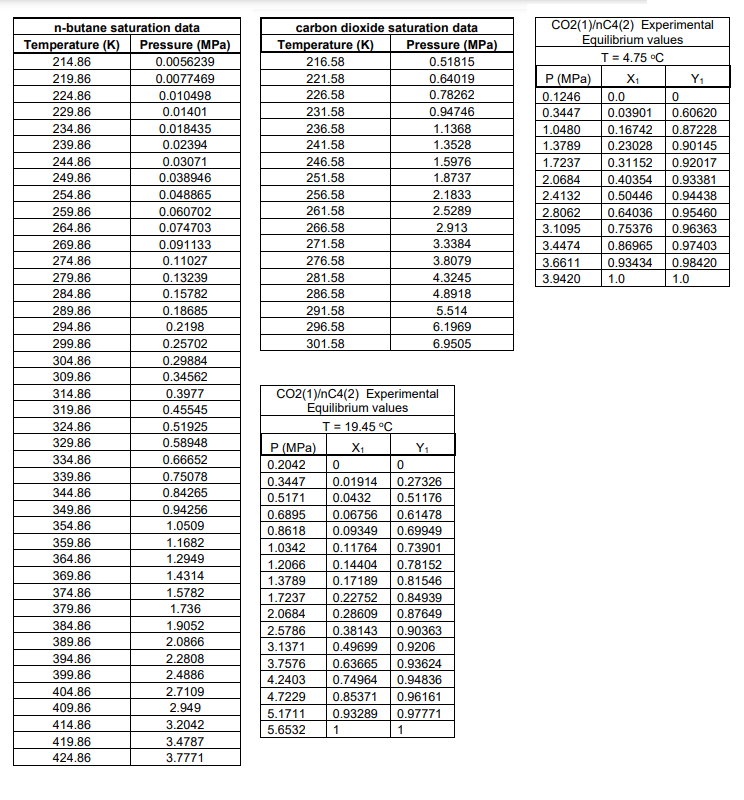
7 2. For the system carbon dioxide (1)-butane(2) make a plot P[MPa] versus X1,Y, for the isotherms given. Compare the results obtained if the Raoult's model was used. From the Raoult's model plot, what could you conclude about this system? ANT2 In PST = ANTI + (T + ANT3) + (ANT4 *T')+(ANT 5* In(T))+(ANT6*TANT) (Eq.1) where; ANT; = constants for each substance T = Temperature, K PSAT = Vapor pressure, Pa Table 1: Antoine constants for carbon dioxide and normal butane Antoine Constant ANTI ANT2 ANT3 ANT4 ANTS ANTO ANTO Tmin (K (K) carbon dioxide -0.52840260 E+04 0.30915310 E+06 0.11114140 E+03 -0.10099290 E+01 0.85020940 E+03 0.18866410 E-14 0.60000000 E+01 0.16731000 E+03 0.30420000 E+03 n-butane -0.20775660 E+04 0.13037520E+06 0.12967170 E+03 -0.40673270 E+00 0.33409570 E+03 0.11135780 E-06 0.30000000 E+01 0.17008000 E+03 0.42520000 E+03 Tres 3. From the experimental equilibrium values for carbon dioxide(1)-butane(2) at 19.45 C find "ki" using the method "O-O" and the Redlich-Kwong EOS. Use the P-T-X1-Y1 at 1.2066 MPa for your calculations. 4. Calculate "ki" using Raoult's Law for the previous P-T-X -Y, values. 5. Find k." experimental for the same P-T-X-Yvalues. 6. Compare the values of k obtained in 3,4,and 5. 7. Plot a P versus T diagram showing the vapor pressure values for both components and the critical locus. From the P-x,y diagram of the system CO2 (1) n-butane (2) plot isopleths at X1 = 0.4 and X1 = 0.6 carbon dioxide saturation data Temperature (K) Pressure (MPa) 216.58 0.51815 221.58 0.64019 226.58 0.78262 231.58 0.94746 236.58 1.1368 241.58 1.3528 246.58 1.5976 251.58 1.8737 256.58 2.1833 261.58 2.5289 266.58 2.913 271.58 3.3384 276.58 3.8079 281.58 4.3245 286.58 4.8918 291.58 5.514 296.58 6.1969 301.58 6.9505 CO2(1)C4(2) Experimental Equilibrium values T = 4.75 C P (MPa) X1 Y1 0.1246 0.0 0 0.3447 0.03901 0.60620 1.0480 0.16742 0.87228 1.3789 0.23028 0.90145 1.7237 0.31152 0.92017 2.0684 0.40354 0.93381 2.4132 0.50446 0.94438 2.8062 0.64036 0.95460 3.1095 0.75376 0.96363 3.4474 0.86965 0.97403 3.6611 0.93434 0.98420 3.9420 1.0 1.0 n-butane saturation data Temperature (K) Pressure (MPa) 214.86 0.0056239 219.86 0.0077469 224.86 0.010498 229.86 0.01401 234.86 0.018435 239.86 0.02394 244.86 0.03071 249.86 0.038946 254.86 0.048865 259.86 0.060702 264.86 0.074703 269.86 0.091133 274.86 0.11027 279.86 0.13239 284.86 0.15782 289.86 0.18685 294.86 0.2198 299.86 0.25702 304.86 0.29884 309.86 0.34562 314.86 0.3977 319.86 0.45545 324.86 0.51925 329.86 0.58948 334.86 0.66652 339.86 0.75078 344.86 0.84265 349.86 0.94256 354.86 1.0509 359.86 1.1682 364.86 1.2949 369.86 1.4314 374.86 1.5782 379.86 1.736 384.86 1.9052 389.86 2.0866 394.86 2.2808 399.86 2.4886 404.86 2.7109 409.86 2.949 414.86 3.2042 419.86 3.4787 424.86 3.7771 CO2(1)/C4(2) Experimental Equilibrium values T = 19.45 C P (MPa) X1 Y 0.2042 0 0 0.3447 0.01914 0.27326 0.5171 0.0432 0.51176 0.6895 0.06756 0.61478 0.8618 0.09349 0.69949 1.0342 0.11764 0.73901 1.2066 0.14404 0.78152 1.3789 0.17189 0.81546 1.7237 0.22752 0.84939 2.0684 0.28609 0.87649 2.5786 0.38143 0.90363 3.1371 0.49699 0.9206 3.7576 0.63665 0.93624 4.2403 0.74964 0.94836 4.7229 0.85371 0.96161 5.1711 0.93289 0.97771 5.6532 1 1








