Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Answer the following questions Looking at the graphs below Below are diagrams that show the energy relationships of three different reactions: reaction X, Y, and
Answer the following questions Looking at the graphs below 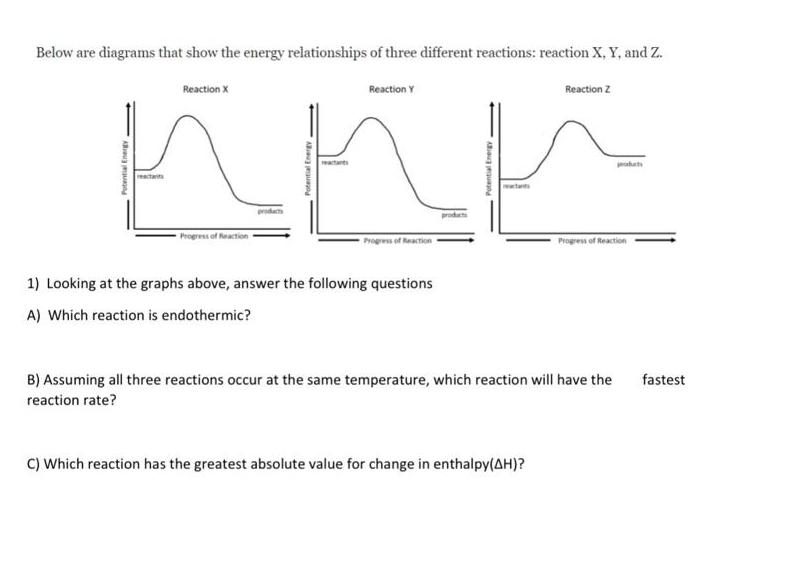
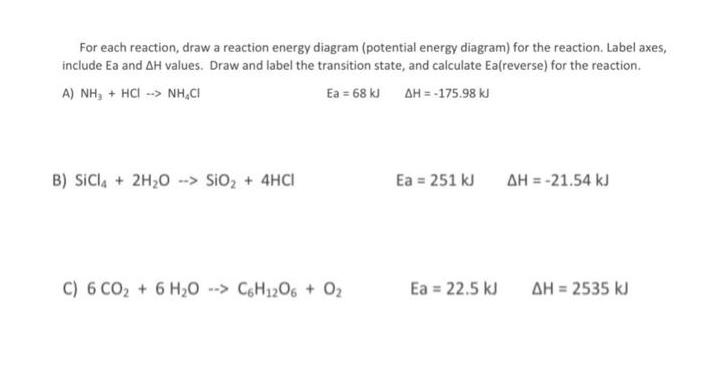
Below are diagrams that show the energy relationships of three different reactions: reaction X, Y, and Z. Reaction Y actants Progress of Reaction Reaction X Progress of Reaction 1) Looking at the graphs above, answer the following questions A) Which reaction is endothermic? Reaction Z C) Which reaction has the greatest absolute value for change in enthalpy(AH)? Progress of Reaction B) Assuming all three reactions occur at the same temperature, which reaction will have the fastest reaction rate?
Step by Step Solution
★★★★★
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Lets start by addressing the questions from the first image where youve provided three reaction energy diagrams for reactions X Y and Z A To determine which reaction is endothermic we look at the ener...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


