Question
Using the critical point, PC = 80.0 bar and TC = 512 K, what is the vapor pressure of methanol at 375 K? (Answer in
Using the critical point, PC = 80.0 bar and TC = 512 K, what is the vapor pressure of methanol at 375 K? (Answer in bar.)
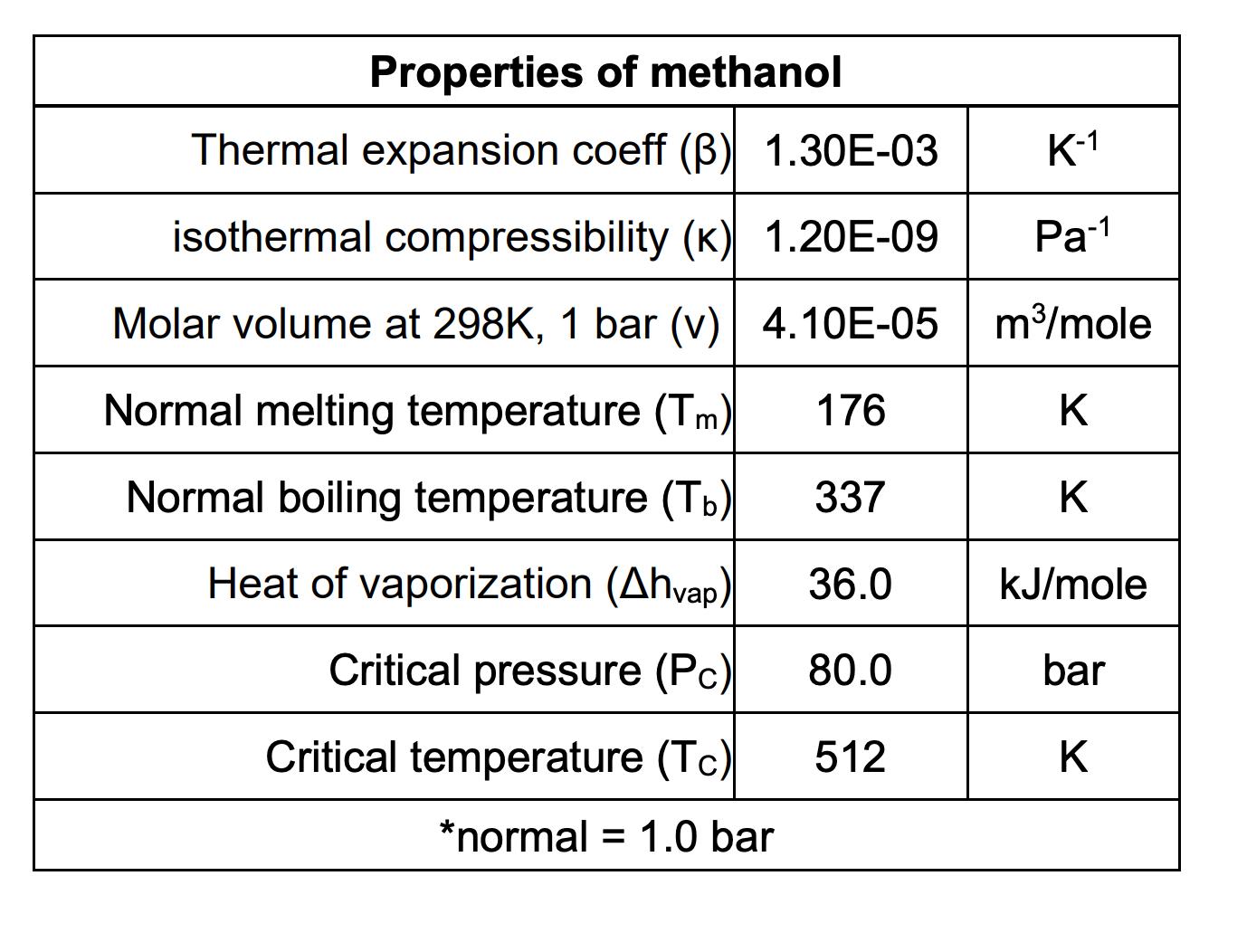
Properties of methanol Thermal expansion coeff () 1.30E-03 isothermal compressibility (K) 1.20E-09 Molar volume at 298K, 1 bar (v) 4.10E-05 K-1 Pa-1 m/mole Normal melting temperature (Tm) 176 K Normal boiling temperature (Tb) 337 K Heat of vaporization (Ahvap) 36.0 kJ/mole Critical pressure (Pc) 80.0 bar Critical temperature (Tc) 512 K *normal = 1.0 bar
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



