Answered step by step
Verified Expert Solution
Question
1 Approved Answer
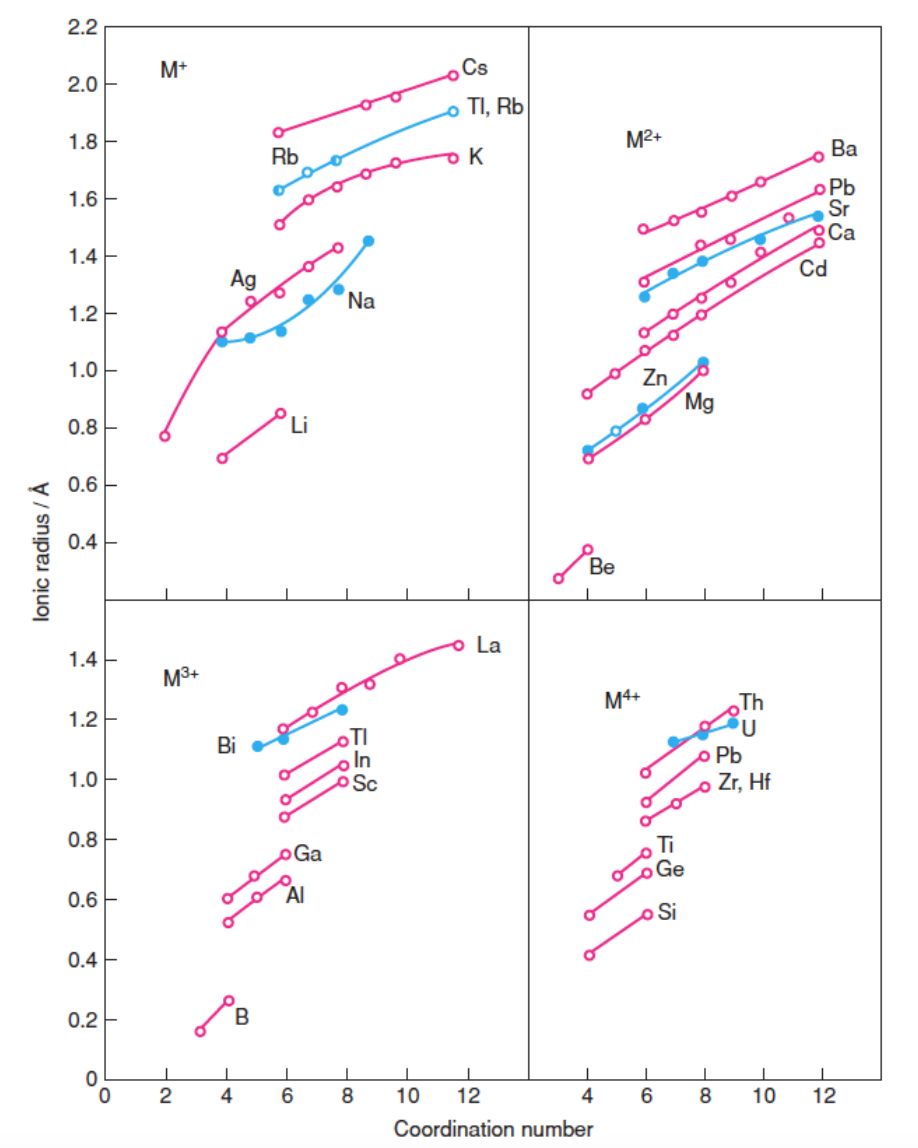
Using the data reported in the figure below, explain: a ) how ionic radii change with atomic number for s and p - block elements;
Using the data reported in the figure below, explain:
a how ionic radii change with atomic number for s and pblock elements;
b how radii change for an isoelectronic cation series select one series at your will;
c for any element with more than one oxidation state, describe how cation radius
changes with increasing oxidation number;
d how the cation radius changes with coordination number.
Consider the ShannonPrewitt model and explain which contribution it provides to rationalize the
trends reported above.
Values of radii on the ordinate are expressed in angstrom, m
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


