Question
Using the Energy Balance with the Ideal Gas Model 3.81 Ammonia is contained in a rigid, well-insulated container. The initial pressure is 138 kPa,
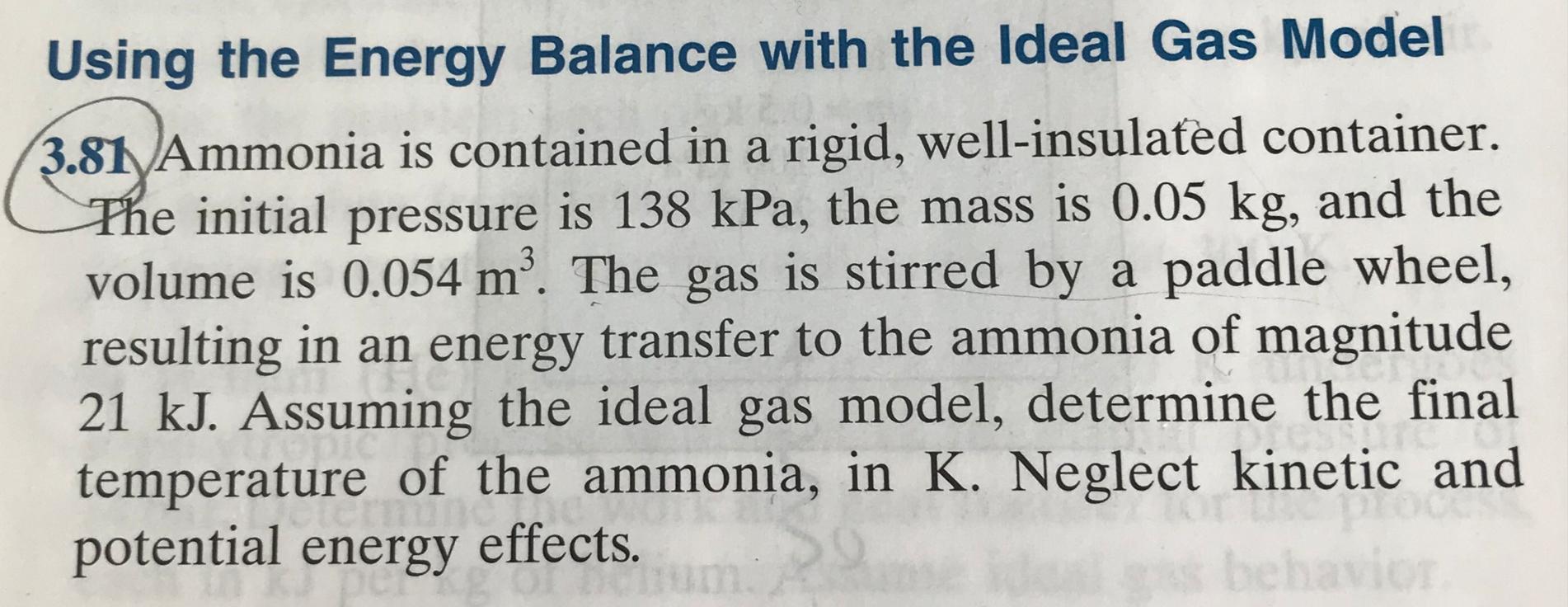
Using the Energy Balance with the Ideal Gas Model 3.81 Ammonia is contained in a rigid, well-insulated container. The initial pressure is 138 kPa, the mass is 0.05 kg, and the volume is 0.054 m. The gas is stirred by a paddle wheel, resulting in an energy transfer to the ammonia of magnitude 21 kJ. Assuming the ideal gas model, determine the final ting temperature of the ammonia, in K. Neglect kinetic and Thun potential energy effects. behavior
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
Given that rigid well insulated container P 138kPa 0054m 1 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Vector Mechanics for Engineers Statics and Dynamics
Authors: Ferdinand Beer, E. Russell Johnston Jr., David Mazurek, Phillip Cornwell, Brian Self
11th edition
73398241, 978-0073398242
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



