Answered step by step
Verified Expert Solution
Question
1 Approved Answer
using the procedure complete the graph the table not the graph 5 Chemistry 113L Name Prelab Worksheet Method of Initial Rates Keep a copy for
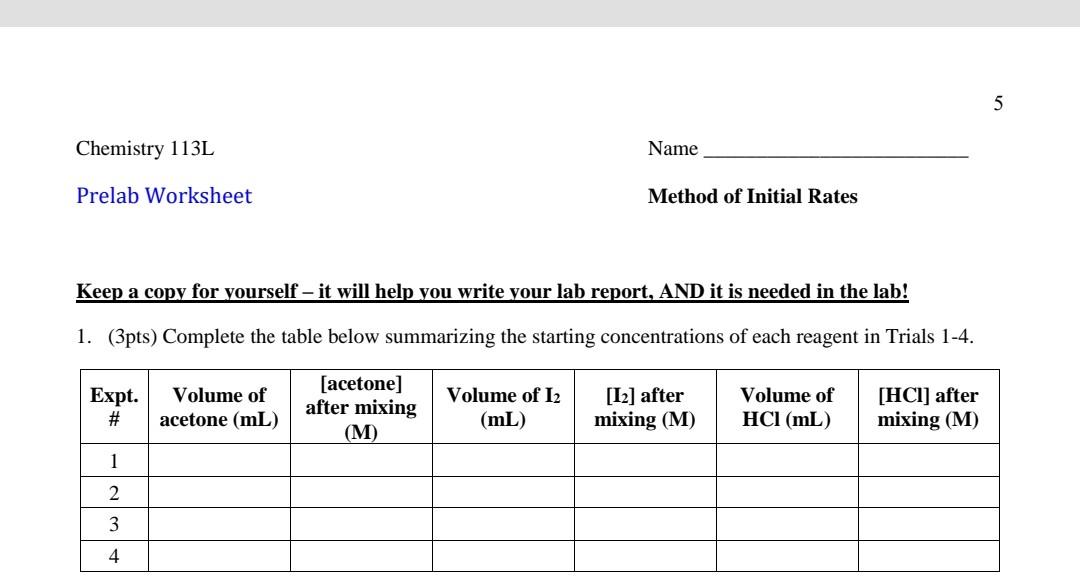

using the procedure complete the graph
the table not the graph
5 Chemistry 113L Name Prelab Worksheet Method of Initial Rates Keep a copy for yourself - it will help you write your lab report, AND it is needed in the lab! 1. (3pts) Complete the table below summarizing the starting concentrations of each reagent in Trials 1-4. Expt. # Volume of acetone (mL) [acetone] after mixing (M) Volume of 12 (mL) [12] after mixing (M) Volume of HCl (mL) [HCI) after mixing (M) 1 2 3 4 Procedure Waste: Do not throw solutions containing acetone or iodine down the drain. Collect all waste in a GLASS beaker, and then pour it into the designated waste bottle in the hood. Safety and Handling: The iodine solution will color the skin. Acetone is flammable. There should be no open flames in the room. Acetone also dissolves plastic cups, so keep it in a glass container. Obtain three clean, dry Dixie cups, four 1 mL syringes, and one well plate. Fill each cup with 5 mL of one of the following: 0.0050 M iodine, 1.0 M HCl and deionized water. Label the contents of each cup with a marker. Additionally, place 5 mL of 4.0 M acetone in a small glass beaker or graduated cylinder: do NOT place it in a plastic cup, as it will dissolve the cup. Keep the acetone and iodine solutions covered with small watch glasses or inverted beakers to limit evaporation. Label one syringe for each solution, and do not mix up the syringes. Clean each syringe by filling it with a small amount of the desired solution, then disposing of that rinse in your waste beaker. For Experiment 1, use your syringes to measure out 0.20 mL of acetone solution, 0.20 mL of 1.0 M HCl, and 0.40 mL of deionized water. Be sure to eliminate all air from the syringe to have an accurate volume measurement. Mix these portions in one empty well of your well plate. Clean and dry a stirring rod; then, measure 0.20 mL of the iodine solution into the last syringe. Note the time on a watch or wall clock to the nearest second and inject the iodine solution into the well plate. Mix the contents of the well gently using the stirring rod. Continue mixing until the last trace of color disappears, and note the reaction time. Dispose of the product solution in a waste beaker at your bench. Repeat twice for a total of three trials. Note that the total volume of the reaction mixture is 1.0 mL. For Experiment 2, set up another reaction mixture in which the volume of acetone is doubled (0.40 mL) and the volume of water, HCl and iodine is 0.20 mL. Combine the reagents in the same order as in the first experiment: acetone, HCl and water are mixed together first; then add iodine to start the reaction. Repeat twice. For Experiment 3, the amount of HCl solution is doubled. Combine 0.20 mL of acetone, 0.40 mL of HCl and 0.20 mL of water together, and then add 0.20 mL of iodine. Repeat twice. For Experiment 4, double the concentrations of acetone and iodine. Combine 0.40 mL of acetone with 0.20 mL of HCl, and then add 0.40 mL of iodine. Repeat twiceStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


