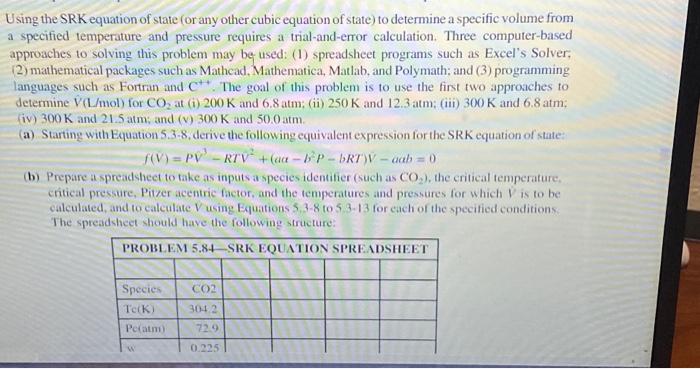
Using the SRK equation of state (or any other cubic equation of state) to determine a specific volume from a specified temperature and pressure requires a trial-and-error calculation. Three computer-based approaches to solving this problem may be- used: (1) spreadsheet programs such as Excel's Solver. (2) mathematical packages such as Mathcad, Mathematica, Matlab, and Polymath; and (3) programming languages such as Fortran and C++. The goal of this problem is to use the first two approaches to determine V^(L/mol) for CO2 at (i) 200K and 6.8atm; (ii) 250K and 12.3atm; (iii) 300K and 6.8atm; (iv) 300K and 21.5atm; and (v) 300K and 50.0atm. (a) Starting with Equation 5.38, derive the following equivalent expression for the SRK equation of state: f(V)=PV3RTV2+(aab2PbRT)Vaab=0 (b) Prepare a spreadsheet to take as inputs a species identifier (such as CO2 ), the critical temperature. critical pressure, Pizer acentric fictor, and the temperatures and pressures for which V^ is to be ealculated, and to calculate V using Equations 5,38 to 5,3.13 for each of the specified conditions: The spreadsheet should have the following structure: 6.194E Find the missing properties and give the phase of the substance, a. H2Os=1.75 Btu/lbm R, T=150F h=?P=?x= ? b. H2Ou=1350Btu/bm,P=1500lbf in. 2 T=2x=?s= ? 6.200E A piston/cylinder reeeives R-410a at 75 psia and compresses it in a reversible adiabatic process to 300psia,160F. Find the initial temperature Using the SRK equation of state (or any other cubic equation of state) to determine a specific volume from a specified temperature and pressure requires a trial-and-error calculation. Three computer-based approaches to solving this problem may be- used: (1) spreadsheet programs such as Excel's Solver. (2) mathematical packages such as Mathcad, Mathematica, Matlab, and Polymath; and (3) programming languages such as Fortran and C++. The goal of this problem is to use the first two approaches to determine V^(L/mol) for CO2 at (i) 200K and 6.8atm; (ii) 250K and 12.3atm; (iii) 300K and 6.8atm; (iv) 300K and 21.5atm; and (v) 300K and 50.0atm. (a) Starting with Equation 5.38, derive the following equivalent expression for the SRK equation of state: f(V)=PV3RTV2+(aab2PbRT)Vaab=0 (b) Prepare a spreadsheet to take as inputs a species identifier (such as CO2 ), the critical temperature. critical pressure, Pizer acentric fictor, and the temperatures and pressures for which V^ is to be ealculated, and to calculate V using Equations 5,38 to 5,3.13 for each of the specified conditions: The spreadsheet should have the following structure: 6.194E Find the missing properties and give the phase of the substance, a. H2Os=1.75 Btu/lbm R, T=150F h=?P=?x= ? b. H2Ou=1350Btu/bm,P=1500lbf in. 2 T=2x=?s= ? 6.200E A piston/cylinder reeeives R-410a at 75 psia and compresses it in a reversible adiabatic process to 300psia,160F. Find the initial temperature