Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Using the volumes and concentrations given in Table 13-2 of the instructions, calculate the diluted concentration of the hydroxide ion at time zero, [OH]o,
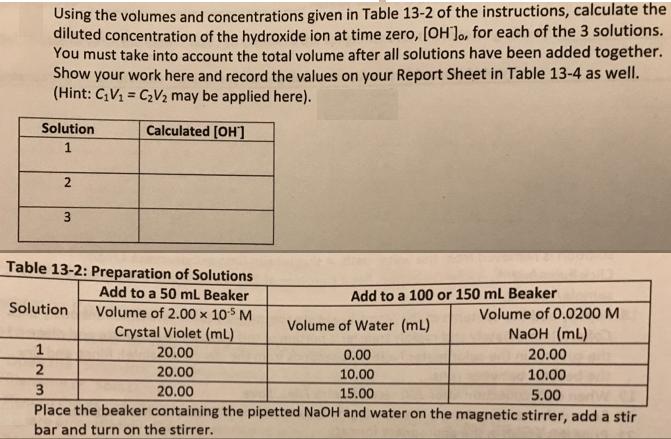
Using the volumes and concentrations given in Table 13-2 of the instructions, calculate the diluted concentration of the hydroxide ion at time zero, [OH]o, for each of the 3 solutions. You must take into account the total volume after all solutions have been added together. Show your work here and record the values on your Report Sheet in Table 13-4 as well. (Hint: CV = C2V2 may be applied here). Solution Calculated [OH'] 1. Table 13-2: Preparation of Solutions Add to a 50 mL Beaker Add to a 100 or 150 mL Beaker Solution Volume of 2.00 x 105 M Crystal Violet (mL) 20.00 Volume of 0.0200 M Volume of Water (mL) NaOH (mL) 20.00 0.00 20.00 10.00 10.00 3 20.00 15.00 5.00 Place the beaker containing the pipetted NaOH and water on the magnetic stirrer, add a stir bar and turn on the stirrer.
Step by Step Solution
★★★★★
3.38 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
C 1 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


