Question
Using the YIELD DATA below determine the following, for a plant designed to produce 150 million liters per year of biofuel: YIELD DATA: 2a) How
Using the YIELD DATA below determine the following, for a plant designed to produce 150 million liters per year of biofuel:
YIELD DATA:
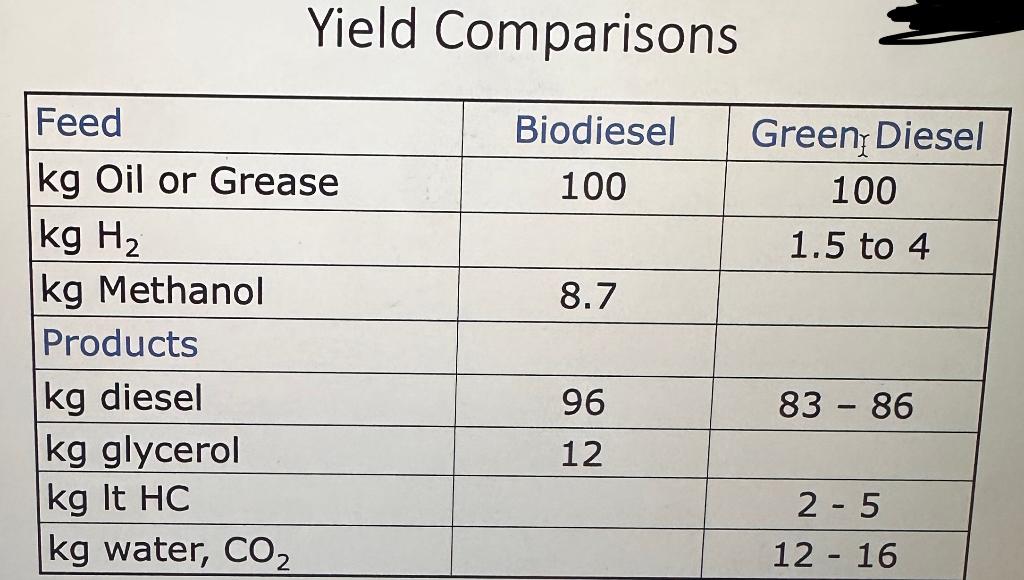
2a) How much vegetable oil (in kg) would be needed to produce the specified quantity of (i) biodiesel (ii) Green diesel (HDRD)? (note that the densities for biodiesel and HDRD are different, at 0.88 and 0.806 kg/L, respectively)
2b) Calculate the quantity of H2 or methanol required. ( H2 = 2.65 wt%)
2c) Calculate the mass of all co-products for (i) biodiesel and (ii) Green diesel. (Products for biodiesel and green diesel are given in the above image)
2d) Assuming canola oil costs 90 cents (Cdn)/L, determine the feedstock cost per liter of biodiesel and green diesel.
2e)Determine total revenues and other expenses, on a Canadian dollar per liter of biofuel basis, assuming an exchange rate of $0.75 USD per CAD, and:
2e) a)Glycerol price is $0.065/lb
b) Light hydrocarbons are priced = propane (same as question 1)
c) Wholesale price of Biodiesel and green diesel=1.945 CAD$/L
d) Hydrogen is priced per question (1), above
e) Methanol price = 1.73$/Gallon
f) Interest, maintenance, depreciation, SG&A, etc. total 8 cents (US)/L for biodiesel, and 12 cents (US)/L for green diesel.
2f) What is the net income/loss for production of biodiesel and green diesel, on a per litre basis.
2g) In the U.S., these fuels would be eligible to earn income on a RIN, currently valued at ~US$2 US gallon, and a Blenders Tax Incentive valued at US$1 per US gallon. What is the profit/loss once these incentives are applied? [Note that fuel sold in California would also receive LCFS credits based upon the carbon intensity of the fuel]
2h) What price of canola oil would generate a break-even situation, i.e., where the net income/loss = zero for parts (2f) and (2g)?
Note: Please assume any missing values, if any
Yield ComparisonsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


