Answered step by step
Verified Expert Solution
Question
1 Approved Answer
using tour data, calculate the moles and mass of acetylsalicylic acid in each tablet (molar mass =180.16g/mol) ConcentrationofNaOHM begin{tabular}{r|c|} cline { 2 - 3 }
using tour data, calculate the moles and mass of acetylsalicylic acid in each tablet (molar mass =180.16g/mol) 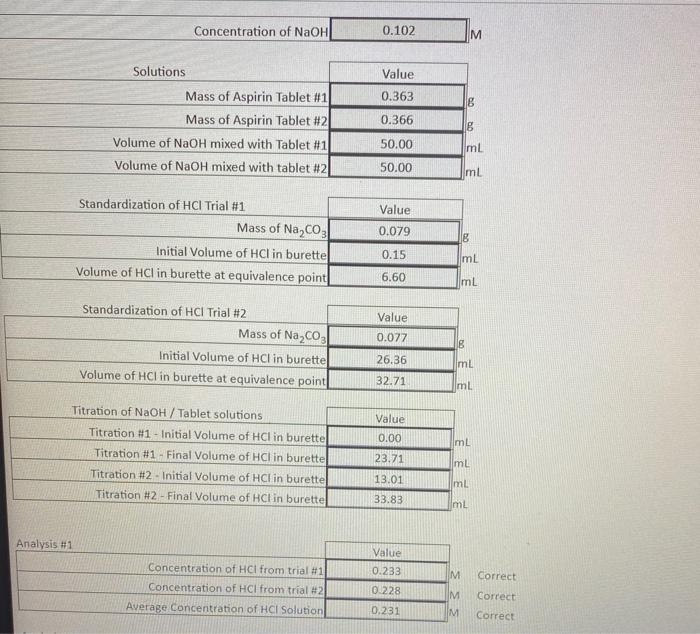
ConcentrationofNaOHM \begin{tabular}{r|c|} \cline { 2 - 3 } \multicolumn{1}{c|}{ Solutions } & Value \\ \hline Mass of Aspirin Tablet \#1 & 0.363 \\ \hline Volume of NaOH mixed with Tablet \#1 & 0.366 \\ \hline Volume of NaOH mixed with tablet \#2 & 50.00 \\ \hline mL \\ mL \\ \hline \end{tabular} \begin{tabular}{|r|c|} \cline { 2 - 3 } Standardization of HCl Trial \#1 & Value \\ \hline Initial Volume of HCl in burette & 0.079 \\ \hline Volume of HCl in burette at equivalence point & 0.15 \\ \hline mL & 6.60 \\ mL \\ \hline \end{tabular} 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


