Answered step by step
Verified Expert Solution
Question
1 Approved Answer
v ICE tables are used for calculating changes in concentration in an equilibrium system. I represents the initial concentration. C the change in concentration between
v 
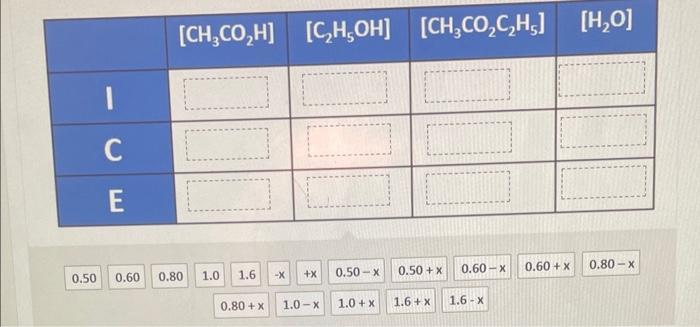
ICE tables are used for calculating changes in concentration in an equilibrium system. I represents the initial concentration. C the change in concentration between the initial value and the equilibrium value, and E the final concentration at equilibrium. The following system is initially at equilibrium. CH3CO2H(g)+C2H5OH(g)CH3CO2C2H5(g)+H2O(g) The concentrations of CH3CO2H4C2H5OH3,CH3CO2C2H5, and H2O in a 1L container are 0.60M,1.0M,1.6M, and 0.50M respectively. 0.20 moles of CH3CO2H are added to the container. Complete the ICE table using values from the following list. Provide your answer below: \begin{tabular}{|c|c|c|c|c|} \hline & {[CH3CO2H]} & {[C2H5OH]} & {[CH3CO2C2H5]} & {[H2O]} \\ \hline 1 & & & & \\ \hline C & & & & \\ \hline E & & & & \\ \hline \end{tabular} 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


