Question
Vapor pressure (mm Hg) 900 800 700 600 500 400 300 200 100 0 10 Carbon disulfide Methanol 20 30 40 50 P Ethanol
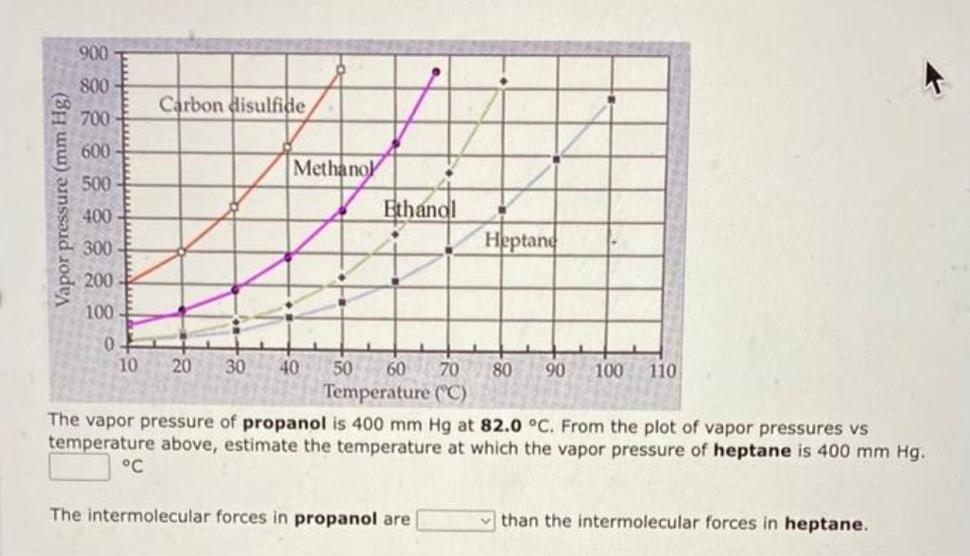
Vapor pressure (mm Hg) 900 800 700 600 500 400 300 200 100 0 10 Carbon disulfide Methanol 20 30 40 50 P Ethanol 60 70 Temperature (C) The intermolecular forces in propanol are Heptane 80 90 100 110 The vapor pressure of propanol is 400 mm Hg at 82.0 C. From the plot of vapor pressures vs temperature above, estimate the temperature at which the vapor pressure of heptane is 400 mm Hg. than the intermolecular forces in heptane.
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials of Materials Science and Engineering
Authors: Donald R. Askeland, Wendelin J. Wright
3rd edition
978-1111576868, 1111576866, 978-1285677620, 1285677625, 978-1111576851
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



