Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Very importnat !! pls help with all parts Which of the following statements is TRUE if K1 ? The rate of the reaction is fast.
Very importnat !! pls help with all parts 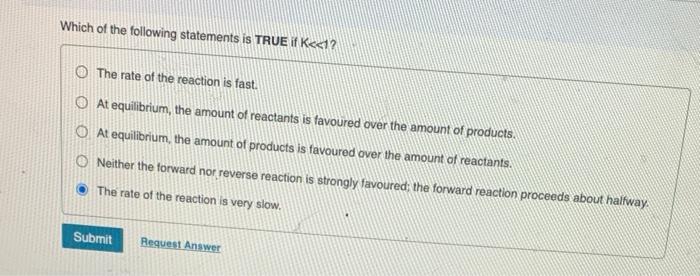
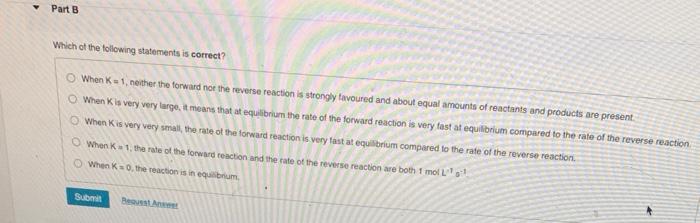
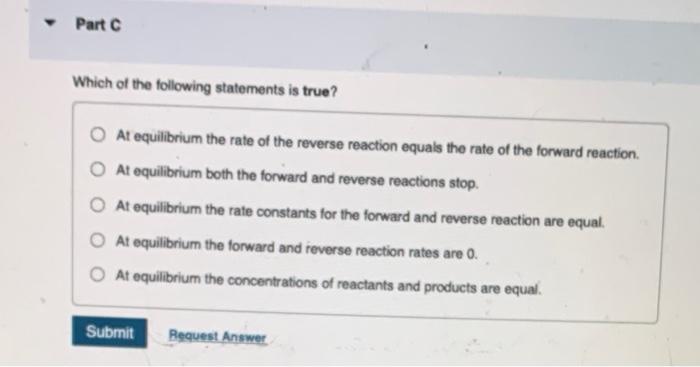
Which of the following statements is TRUE if K1 ? The rate of the reaction is fast. At equilibrium, the amount of reactants is favoured over the amount of products. At equilibrium, the amount of products is favoured over the amount of reactants. Neither the forward nor reverse reaction is strongly favoured; the forward reaction proceeds about halfway. The rate of the reaction is very slow. When K=1, neither the forward noe the reverse reaction is strongly tavoured and about equal amounts of reactants and products are present. When K is very very laroo, it means that at equilibriat the rate of the forward reaction is very fast at equilibrium compared to the rate of the reverse reaction. Which of the following statements is true? At equilibrium the rate of the reverse reaction equals the rate of the forward reaction. At equilibrium both the forward and reverse reactions stop. At equilibrium the rate constants for the forward and reverse reaction are equal. At equilibrium the fonward and reverse reaction rates are 0 . At equilibrium the concentrations of reactants and products are equal 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


