Answered step by step
Verified Expert Solution
Question
1 Approved Answer
VII. An unknown compound D exhibits a strong absorption band in its IR spectrum at 1692 cm-1, The mass spectrum of D shows the molecular
VII. An unknown compound D exhibits a strong absorption band in its IR spectrum at 1692 cm-1, The mass spectrum of D shows the molecular ion at m/e = 150 and a base peak at m/e = 121. If the spectrum
of proton of D is as follows.
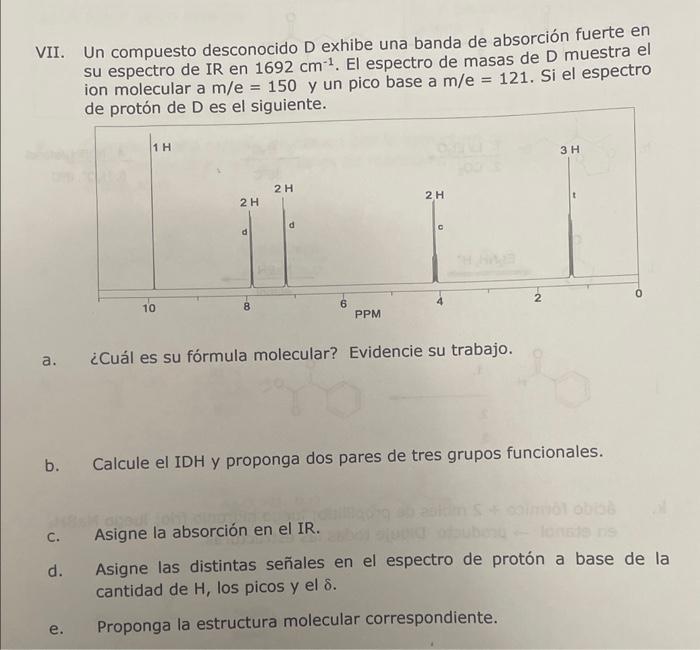
a. What is its molecular formula? Show your work.
b. Calculate the HDI and propose two pairs of three functional groups.
c. Assign the absorption in the IR.
D. Assign the different signals in the proton spectrum based on the amount of H, the peaks, and the &.
E. Propose the corresponding molecular structure.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


