Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Volumetric analysis of natural gas shows the following: 70% CH4, 10% H2, 15% N2, 2% O2 and 3% CO2. This gas enters a combustion
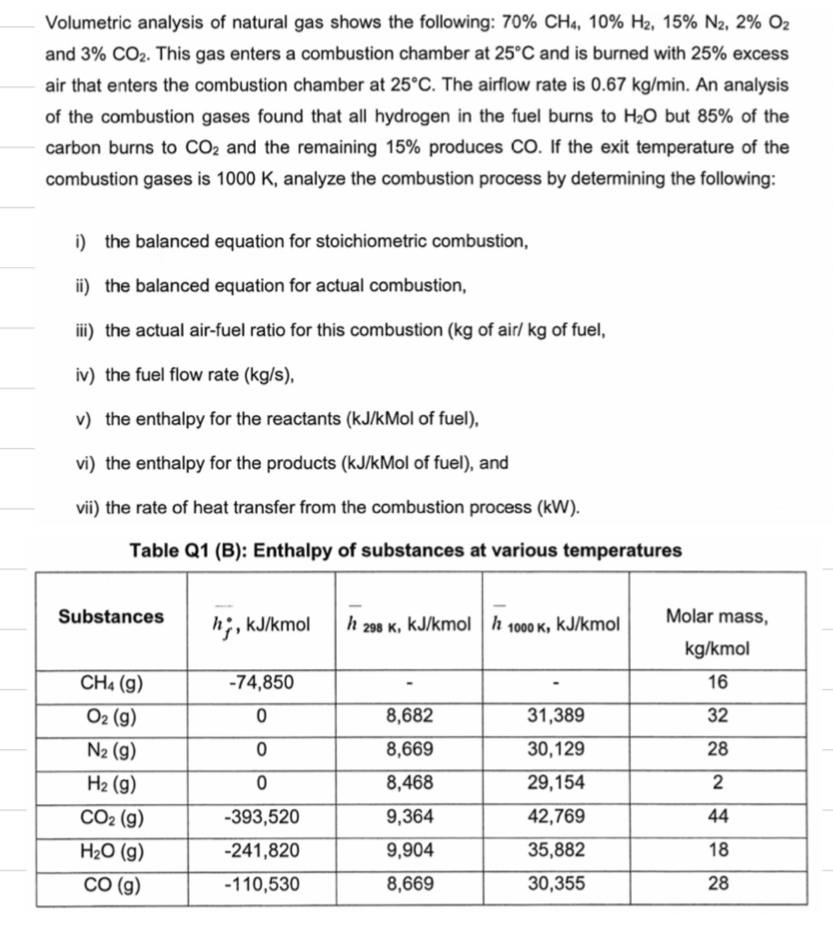
Volumetric analysis of natural gas shows the following: 70% CH4, 10% H2, 15% N2, 2% O2 and 3% CO2. This gas enters a combustion chamber at 25C and is burned with 25% excess air that enters the combustion chamber at 25C. The airflow rate is 0.67 kg/min. An analysis of the combustion gases found that all hydrogen in the fuel burns to H2O but 85% of the carbon burns to CO2 and the remaining 15% produces CO. If the exit temperature of the combustion gases is 1000 K, analyze the combustion process by determining the following: i) the balanced equation for stoichiometric combustion, ii) the balanced equation for actual combustion, iii) the actual air-fuel ratio for this combustion (kg of air/ kg of fuel, iv) the fuel flow rate (kg/s), v) the enthalpy for the reactants (kJ/kMol of fuel), vi) the enthalpy for the products (kJ/kMol of fuel), and vii) the rate of heat transfer from the combustion process (kW). Table Q1 (B): Enthalpy of substances at various temperatures Substances h, kJ/kmol h 298 K, kJ/kmol h 1000 K, kJ/kmol Molar mass, kg/kmol CH4 (g) -74,850 16 O2 (g) 0 8,682 31,389 32 N2 (g) 0 8,669 30,129 28 H2 (g) 0 8,468 29,154 2 CO2 (g) -393,520 9,364 42,769 44 HO (g) -241,820 9,904 35,882 18 CO (g) -110,530 8,669 30,355 28
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


