Answered step by step
Verified Expert Solution
Question
1 Approved Answer
WARNING: Please send your result via the Slack program by a simple message. Please avoid any attachment! Please send me a numerical solution! Deadline: next
WARNING: Please send your result via the Slack program by a simple message. Please avoid
any attachment! Please send me a numerical solution! Deadline: next Monday.
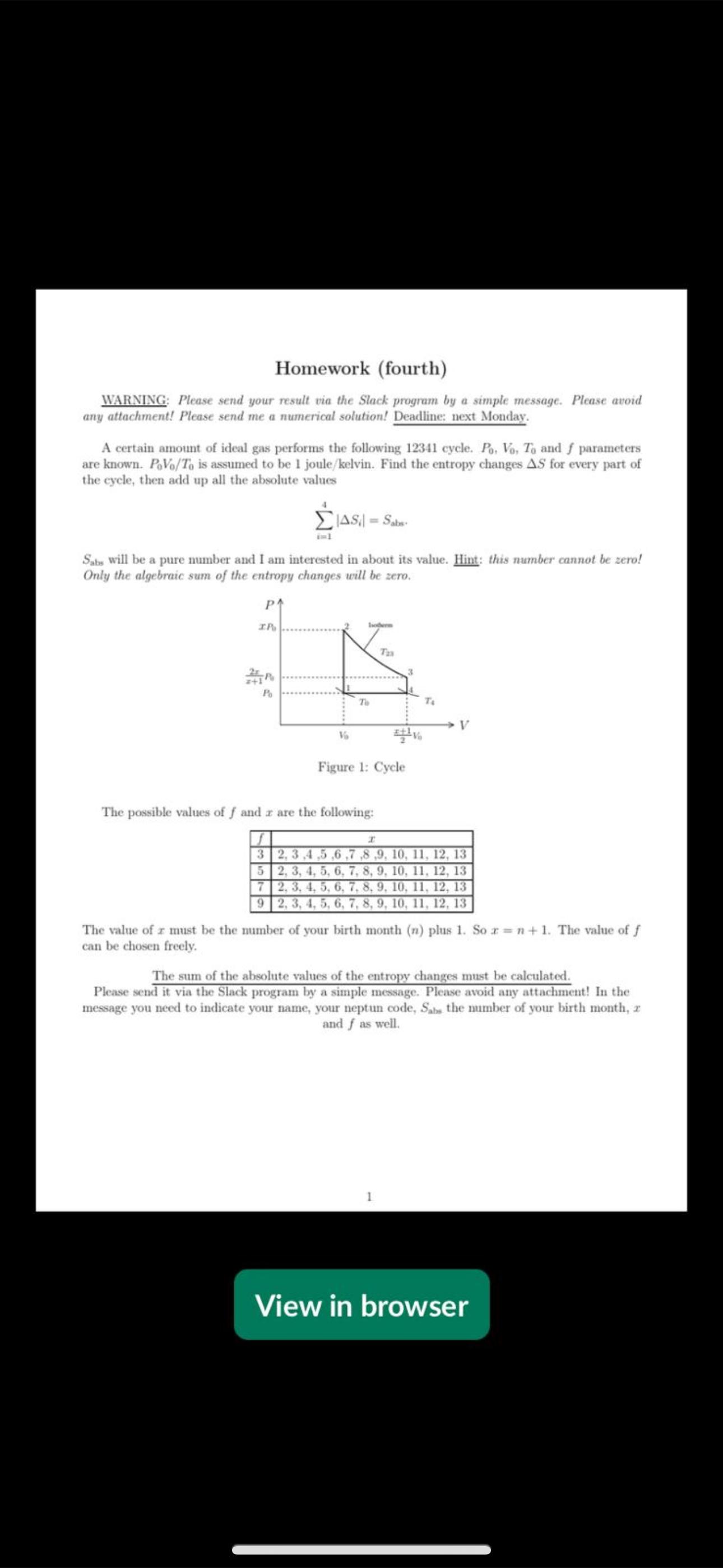
A certain amount of ideal gas performs the following cycle. and parameters
are known. is assumed to be joulekelvin Find the entropy changes for every part of
the cycle, then add up all the absolute values
will be a pure number and I am interested in about its value. Hint: this number cannot be zero!
Only the algebraic sum of the entropy changes will be zero.
The possible values of and are the following:
The value of must be the number of your birth month plus So The value of
can be chosen freely.
The sum of the absolute values of the entropy changes must be calculated.
Please send it via the Slack program by a simple message. Please avoid any attachment! In the
message you need to indicate your name, your neptun code, the number of your birth month,
and as well.
cvf cpf as x and f take any value from table
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


