Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Show your work for your calculations. Mass of crucible + lid Mass crucible + lid+ hydrate Mass of hydrate Mass of crucible + lid
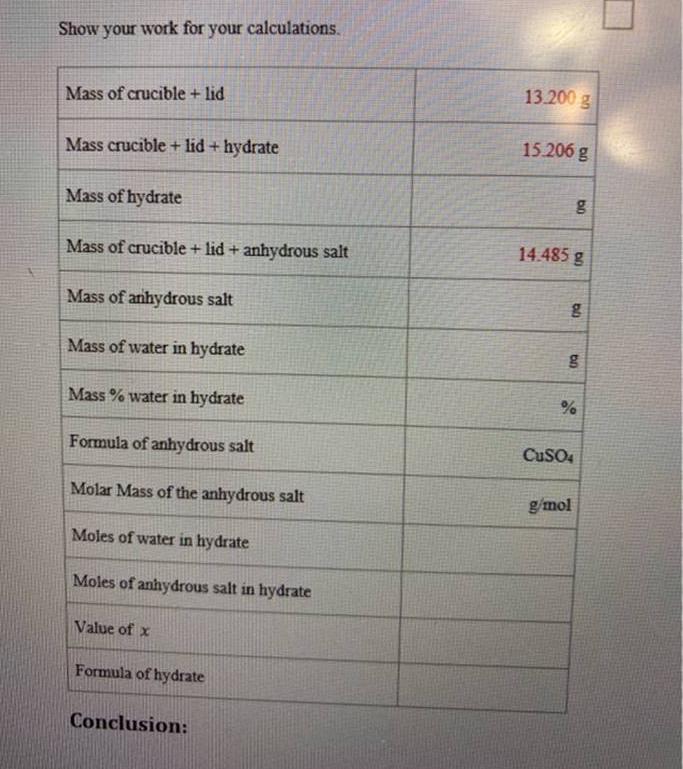

Show your work for your calculations. Mass of crucible + lid Mass crucible + lid+ hydrate Mass of hydrate Mass of crucible + lid + anhydrous salt Mass of anhydrous salt Mass of water in hydrate Mass % water in hydrate Formula of anhydrous salt Molar Mass of the anhydrous salt Moles of water in hydrate Moles of anhydrous salt in hydrate Value of x Formula of hydrate Conclusion: 13.200 g 15.206 g bo 14.485 g g g % CuSO4 g/mol Questions: 1. True or False: The hydrate is the salt that remains after heating. 2. True or False: The anhydrous salt does not contain water. 3. The hydrate salt was heated and the weight after heating was determined to be 3.894. Is this the mass of the hydrate salt or of the anhydrous salt? 4. Given the following grams of the substances, what is the percentage of C in the compound? Mass of C = 2.402 g Mass of the compound = 10.304 g 5. Given the following grams of the elements, what is the empirical formula of the compound? 2.45 g Si 12.4 g Cl
Step by Step Solution
★★★★★
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Here are the stepbystep calculations with explanations Mass of crucible lid 13200 g Mass crucible li...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


