Question
We can draw three inequivalent Lewis structures for the tellurite ion, TeO32. The concepts of formal charge and electronegativity call help us choose the
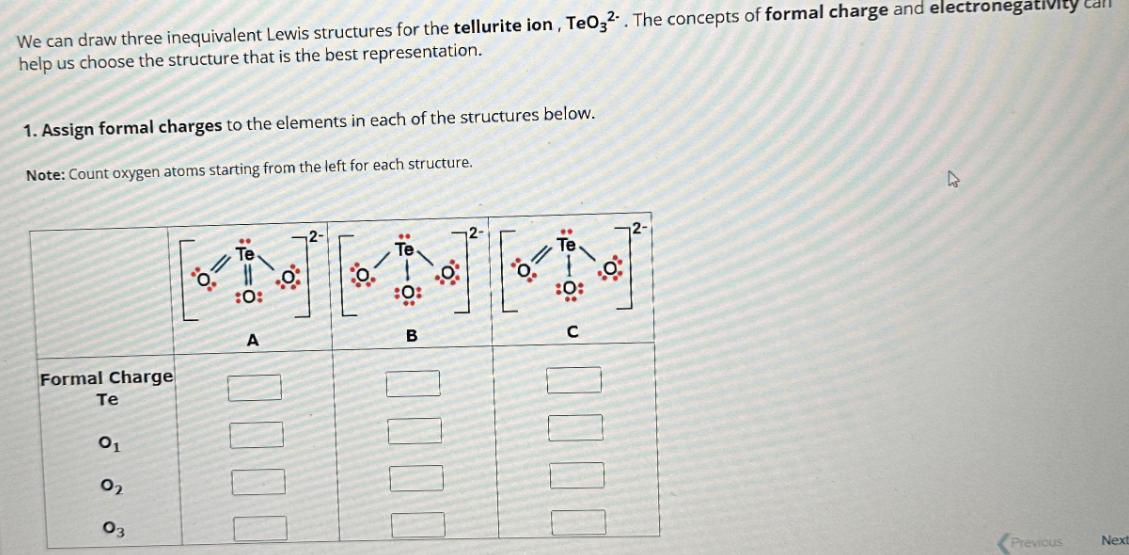
We can draw three inequivalent Lewis structures for the tellurite ion, TeO32. The concepts of formal charge and electronegativity call help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below. Note: Count oxygen atoms starting from the left for each structure. Formal Charge Te 01 0 03 :0: A 0% 2- :0: B :0: 0% D Previous Next
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus For Business, Economics And The Social And Life Sciences
Authors: Laurence Hoffmann, Gerald Bradley, David Sobecki, Michael Price
11th Brief Edition
978-0073532387, 007353238X
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



