Answered step by step
Verified Expert Solution
Question
1 Approved Answer
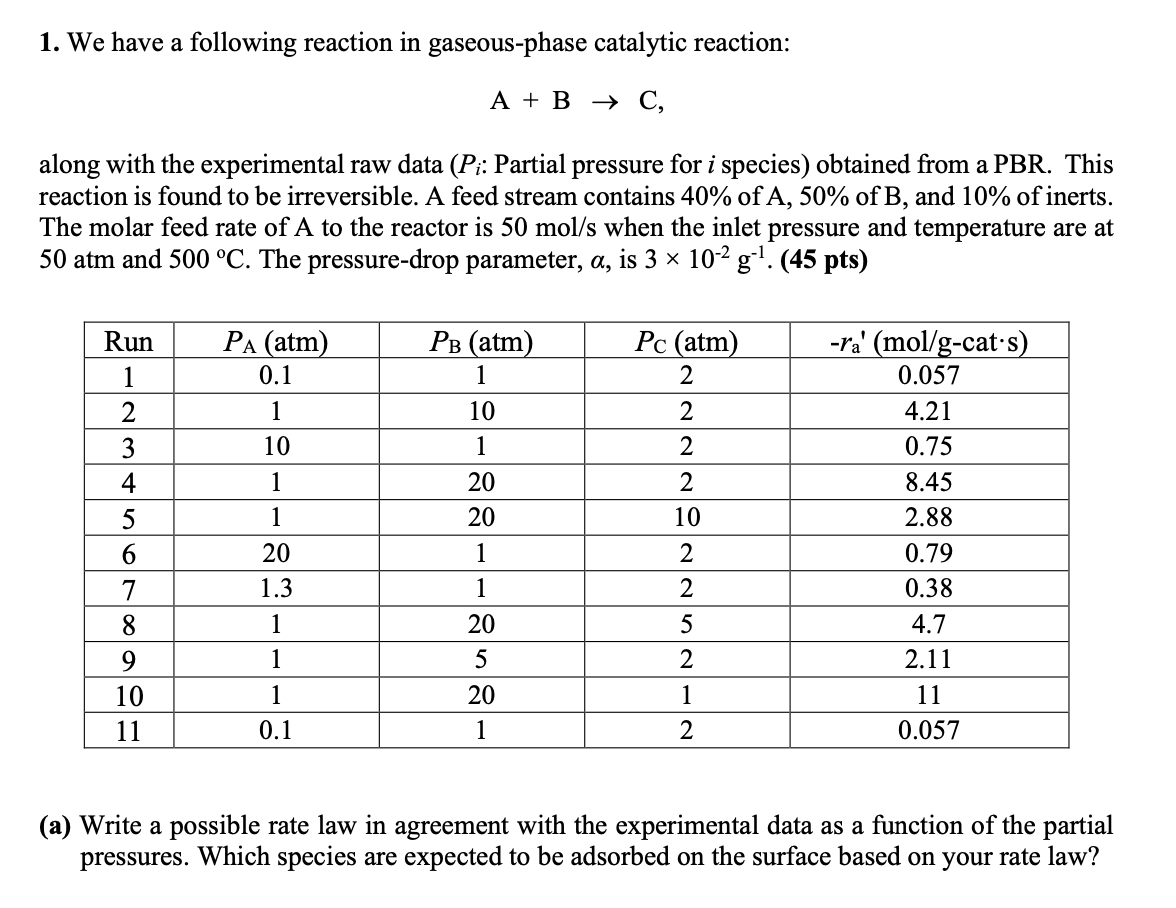
We have a following reaction in gaseous - phase catalytic reaction: A + B C , - > along with the experimental raw data (
We have a following reaction in gaseousphase catalytic reaction:
A B C
along with the experimental raw data Pi: Partial pressure for i species obtained from a PBR This
reaction is found to be irreversible. A feed stream contains of A of B and of inerts.
The molar feed rate of A to the reactor is mols when the inlet pressure and temperature are at
atm and oC The pressuredrop parameter, alpha is times g
a Write a possible rate law in agreement with the experimental data as a function of the partial
pressures. Which species are expected to be adsorbed on the surface based on your rate law? b Using a nonlinear regression method, estimate three rate law parameters appearing on your
rate law obtained in ac Plot and analyze how p X PA PB PC and rA vary as a function of WWe have a following reaction in gaseousphase catalytic reaction:
along with the experimental raw data : Partial pressure for i species obtained from a PBR This reaction is found to be irreversible. A feed stream contains of A of B and of inerts. The molar feed rate of to the reactor is when the inlet pressure and temperature are at atm and The pressuredrop parameter, is
tableRuncat's
a Write a possible rate law in agreement with the experimental data as a function of the partial pressures. Which species are expected to be adsorbed on the surface based on your rate law?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


