website of the article: https://www.nejm.org/doi/full/10.1056ejmoa1403285
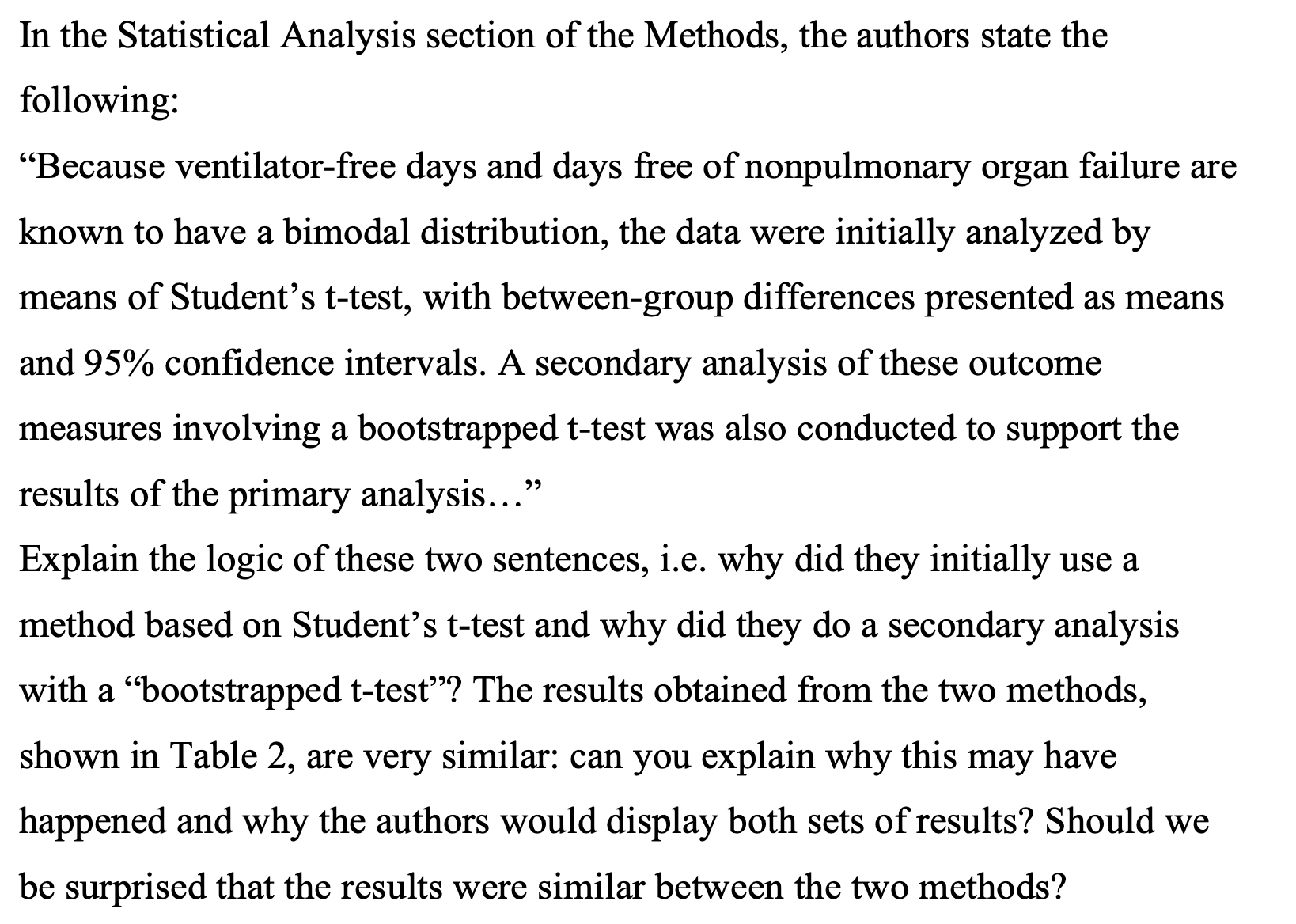
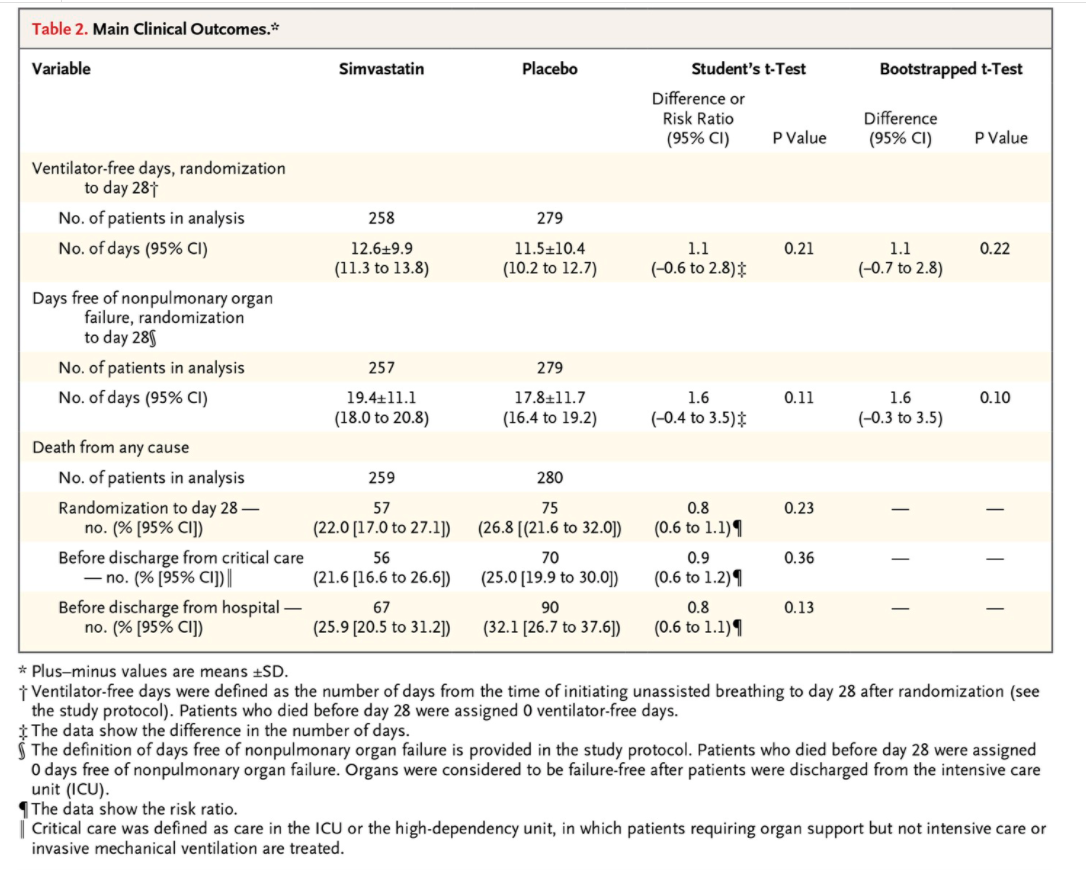
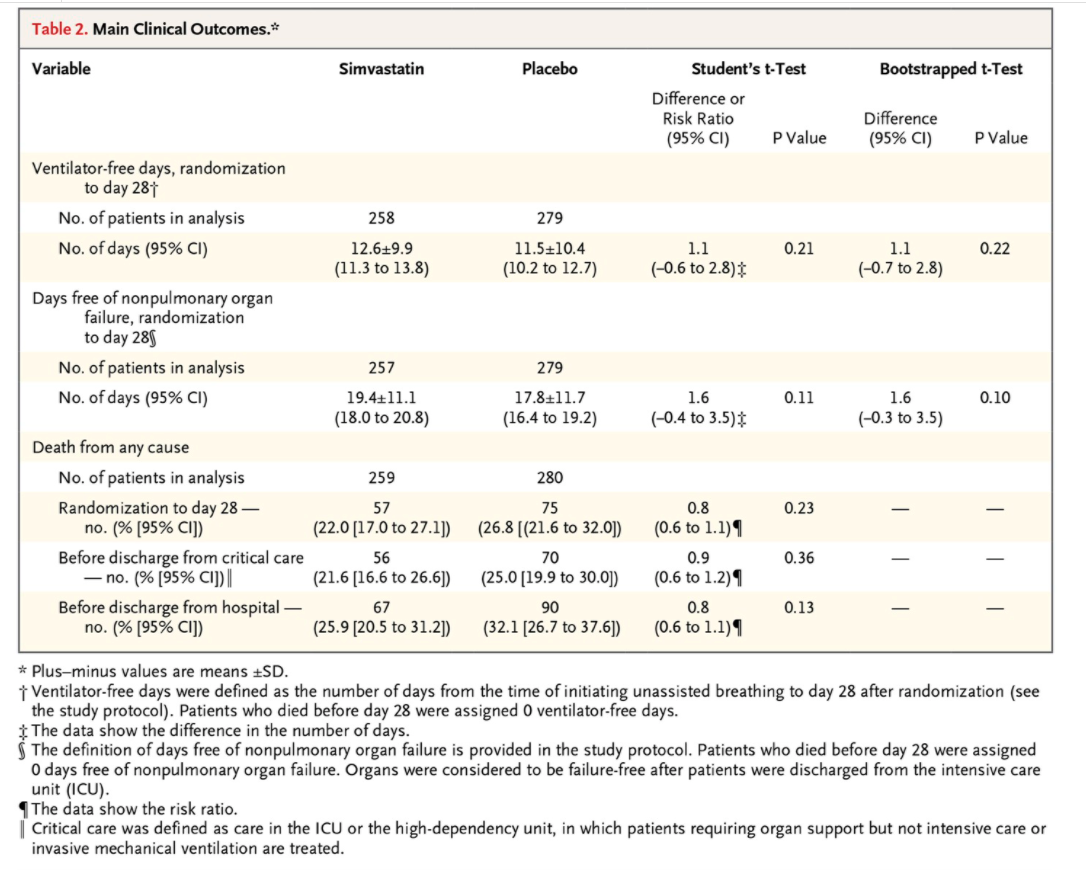
In the Statistical Analysis section of the Methods, the authors state the following: \"Because ventilator-free days and days free of nonpulmonary organ failure are known to have a bimodal distribution, the data were initially analyzed by means of Student's t-test, with between-group differences presented as means and 95% condence intervals. A secondary analysis of these outcome measures involving a bootstrapped ttest was also conducted to support the results of the primary analysis...\" Explain the logic of these two sentences, i.e. why did they initially use a method based on Student's t-test and why did they do a secondary analysis with a \"bootstrapped t-test\"? The results obtained from the two methods, shown in Table 2, are very similar: can you explain why this may have happened and why the authors would display both sets of results? Should we be surprised that the results were similar between the two methods? Table 2. Main Clinical Outcomes.# Variable Simvastatin Placebo Student's t-Test Bootstrapped t-Test Difference or Risk Ratio Difference (95% CI) P Value (95% CI) P Value Ventilator-free days, randomization to day 281 No. of patients in analysis 258 279 No. of days (95% CI) 12.619.9 11.5+10.4 1.1 0.21 1.1 0.22 (11.3 to 13.8) (10.2 to 12.7) (-0.6 to 2.8) (-0.7 to 2.8) Days free of nonpulmonary organ failure, randomization to day 289 No. of patients in analysis 257 279 No. of days (95% CI) 19.4+11.1 17.8+11.7 1.6 0.11 1.6 0.10 (18.0 to 20.8) (16.4 to 19.2) (-0.4 to 3.5)$ (-0.3 to 3.5) Death from any cause No. of patients in analysis 259 280 Randomization to day 28 - 57 75 0.8 0.23 no. (% [95% CI]) (22.0 [17.0 to 27.1]) (26.8 [(21.6 to 32.0]) (0.6 to 1.1) 1 Before discharge from critical care 56 70 0.9 0.36 - no. (% [95% CI]) | (21.6 [16.6 to 26.6]) (25.0 [19.9 to 30.0]) (0.6 to 1.2) 1 Before discharge from hospital - 67 90 0.8 0.13 no. (% [95% CI]) (25.9 [20.5 to 31.2]) (32.1 [26.7 to 37.6]) (0.6 to 1.1) 1 * Plus-minus values are means +SD. i Ventilator-free days were defined as the number of days from the time of initiating unassisted breathing to day 28 after randomization (see the study protocol). Patients who died before day 28 were assigned 0 ventilator-free days. $ The data show the difference in the number of days. The definition of days free of nonpulmonary organ failure is provided in the study protocol. Patients who died before day 28 were assigned 0 days free of nonpulmonary organ failure. Organs were considered to be failure-free after patients were discharged from the intensive care unit (ICU). The data show the risk ratio. Critical care was defined as care in the ICU or the high-dependency unit, in which patients requiring organ support but not intensive care or invasive mechanical ventilation are treated