Answered step by step
Verified Expert Solution
Question
1 Approved Answer
wgolo Wor... increase 50 X=70(T-3037/[(50-10(T-300)] REACTION ENGINEERING AL REACTION ENGINEERING 2-2 11 April - 17 Aprilmid rxn2 Given the following reaction for a feed at
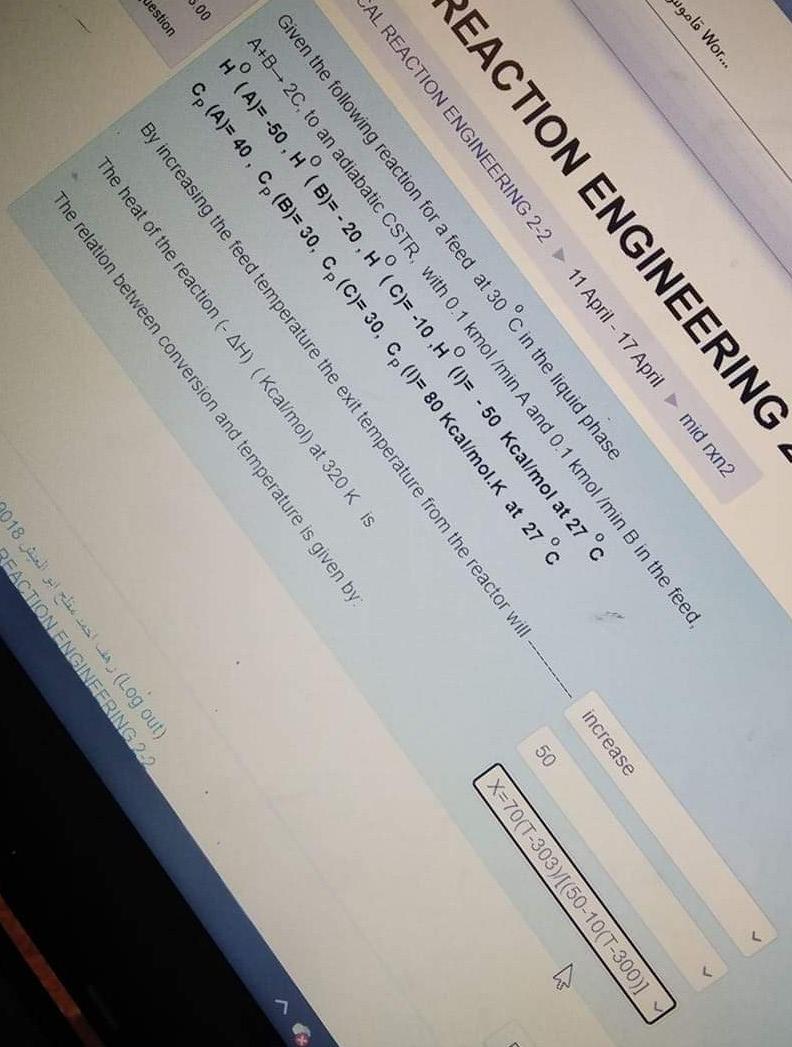
wgolo Wor... increase 50 X=70(T-3037/[(50-10(T-300)] REACTION ENGINEERING AL REACTION ENGINEERING 2-2 11 April - 17 Aprilmid rxn2 Given the following reaction for a feed at 30 C in the liquid phase A+B-2C, to an adiabatic CSTR, with 0.1 kmol /min A and 0.1 kmol/min B in the feed, H (A)= -50, H (B)= - 20, H (C)=-10,H (1)= - 50 Kcal/mol at 27 c Cp (A)= 40, Cp (B)= 30, Cp (C)= 30, CP (1= 80 Kcal/mol.K at 27C 00 estion By increasing the feed temperature the exit temperature from the reactor will The heat of the reaction (- AH) (Kcal/mol) at 320 K is The relation between conversion and temperature is given by (000Ut) 018 PEACTION ENGINEERING:2:22
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


