Question
What additions must you make to the cell for it to generate astandard EMF? (Check all that apply) 1.) You should apply the initial EMF
What additions must you make to the cell for it to generate astandard EMF?
(Check all that apply)
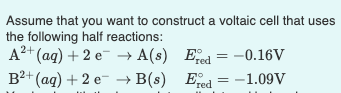
1.) You should apply the initial EMF to the electrode
2.) You should add enough salt containingB+ into the glass containing the electrode B(s) toproduce a 1 M B2+ solution
3.) You should add enough salt containingA2+ into the glass containing the electrode B(s) toproduce a 1 M A2+ solution
4.) You should add enough salt containingB2+ containing the electrode A(s) to produce a 1 MB2+ solution
5.) You should add the salt bridge
6.) You should add enough salt containingA2+ into the glass containing the electrode A(s) toproduce 1 M A2+ solution
Part B:
Which electrode functions as the cathode, and in which directiondo electrons move through the external circuit?
A.) Electrode A functions as the cathode, and electrons flowfrom electrode B to electrode A
B.) Electrode B functions as the cathode, and electrons flowfrom electrode A to electrode B
C.) Electrode A functions as the cathode, and electrons flowfrom electrode A to electrode B
D.) Electrode B functions as the cathode, and electrons flowfrom electrode B to electrode A
Assume that you want to construct a voltaic cell that uses the following half reactions: 2+ A+ (aq) + 2 e A(s) B+ (aq) + 2e B(s) Ered = -0.16V Ered=-1.09V
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below Answer Part A 2 5 6 In order to construct a standard electrochemical cell several additional components must be added to the cell The first ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


